Polycystic ovary syndrome and metabolic syndrome
Syndrom polycystických ovarií a metabolický syndrom
Syndrom polycystických ovarií (PCOS) tvoří heterogenní skupinu onemocnění, jejímiž hlavními klinickými znaky jsou nepravidelnost menstruačního cyklu, snížená fertilita a hyperandrogenismus. Prevalence PCOS je závislá na etnické příslušnosti pacientky, na environmentálních a genetických faktorech a také na kritériích, která tento syndrom definují. Na druhé straně představuje metabolický syndrom soubor metabolických poruch zahrnujících hlavně abdominální obezitu, inzulinovou rezistenci, narušený metabolismus glukózy, hypertenzi a dyslipidémii. Tyto přidružené poruchy přímo úměrně zvyšují riziko vzniku diabetes mellitus typu 2 (DMT2), onemocnění koronárních tepen (CHD), onemocnění kardiovaskulárního aparátu (CVD) a karcinomu endometria. Mnoho pacientek s PCOS má znaky metabolického syndromu, jako je viscerální obezita, hyperinzulinémie a inzulinová rezistence. Pacientky s PCOS jsou ve vysokém riziku rozvoje kardiovaskulárních chorob (CVD), cukrovky typu 2 (DMT2) a gynekologických nádorů, hlavně karcinomu endometria. Výskyt metabolického syndromu je taktéž častější u neplodných žen s PCOS. Cílem tohoto přehledového článku je poskytnout jasné a nejnovější informace o PCOS a o jeho souvislostech s metabolickým syndromem a o možných interakcích mezi různými metabolickými poruchami.
klíčová slova:
syndrom polycystických ovarií, metabolický syndrom, inzulinová rezistence, hypertenze
Authors:
Aus Tariq Ali
Authors‘ workplace:
Department of Chemical Pathology, Tygerberg Hospital and Stellenbosch Medical School, Cape Town, South Africa
Published in:
Ceska Gynekol 2015; 80(4): 279-289
Overview
Polycystic ovary syndrome (PCOS) is a heterogeneous disorder, where the main clinical features include menstrual irregularities, sub-fertility, hyperandrogenism, and hirsutism. The prevalence of PCOS depends on ethnicity, environmental and genetic factors, as well as the criteria used to define it. On the other hand, metabolic syndrome is a constellation of metabolic disorders which include mainly abdominal obesity, insulin resistance, impaired glucose metabolism, hypertension and dyslipidaemia. These associated disorders directly increase the risk of Type 2 diabetes mellitus (DMT2), coronary heart disease (CHD), cardiovascular diseases (CVD) and endometrial cancer. Many patients with PCOS have features of metabolic syndrome such as visceral obesity, hyperinsulinaemia and insulin resistance. These place patients with PCOS under high risk of developing cardiovascular disease (CVD), Type 2 diabetes (DMT2) and gynecological cancer, in particular, endometrial cancer. Metabolic syndrome is also increased in infertile women with PCOS. The aim of this review is to provide clear and up to date information about PCOS and its relationship with metabolic syndrome, and the possible interaction between different metabolic disorders.
Keywords:
polycystic ovary syndrome, metabolic syndrome, insulin resistance, hypertension
INTRODUCTION
Polycystic ovary syndrome (PCOS) is one of the most common (if not the most common) endocrinopathy among women of reproductive age. The classic features associated with PCOS include ovarian dysfunction (menstrual irregularities or sub-fertility), hyperandrogenism, and polycystic ovarian morphology [35].Visceral obesity which is believed to be a result of insulin resistance [3] is also common in women with PCOS [29, 33].These two features appear to contribute extensively to the increased incidence of type 2 diabetes mellitus (DMT2) and cardiovascular disease (CVD) in women with PCOS. Metabolic syndrome on the other hand is a constellation of metabolic disorders including mainly abdominal obesity, insulin resistance, impaired glucose metabolism, hypertension and dyslipidaemia [97]. Generally, the prevalence of metabolic syndrome is affected by diet, lifestyle and genetic factors. It is however more prevalent in developed countries than developing countries [37, 82]. An increase in the prevalence of obesity due to the interaction between diet and lifestyle has been clearly seen in immigrants who moved from third world countries (namely Nigeria and Mexico) to developed countries like the USA, UK and Canada. The prevalence of metabolic syndrome is also affected by age progression and is thus more prevalent in older people than younger people [4, 72].
An increase in obesity, in particular visceral obesity [9], impaired glucose tolerance [18, 30], dyslipidaemia [28, 83] and hypertension [6] in patients with PCOS have increased the risk of developing various cardiometabolic diseases [46]. Thus, it has been suggested that PCOS should no longer be considered a purely gynaecological disorder. In parallel, woman with metabolic syndrome usually present with similar features including impaired glucose tolerance, central obesity, hypertension and hypercholesterolaemia [54, 76]. The association of PCOS with metabolic syndrome shows a consequent increase in the long-term risk of DMT2, CVD, and certain types of cancer (namely breast and gynecological cancer). Infertility is increased among patients with PCOS, and is positively associated with metabolic syndrome. This indicates that PCOS carries significant public health implications. The health impact is dependent on the prevalence of metabolic syndrome in patients with PCOS. The prevalence of metabolic syndrome in women with PCOS is ruled by many factors including age, ethnicity, genetics and lifestyle factors (namely physical activity, diet or eating behavior). This review will provide details on the relationship between PCOS and metabolic syndrome.
METHODOLOGY
After an intensive search through PubMed using combinations of specific key words, I have gathered information on all English-language publications on PCOS, metabolic syndrome, PCOS and metabolic syndrome. Articles that were not accessible or not found through the university‘s electronic library were requested directly from the authors. Case reports and studies expressed methodological weaknesses were excluded.
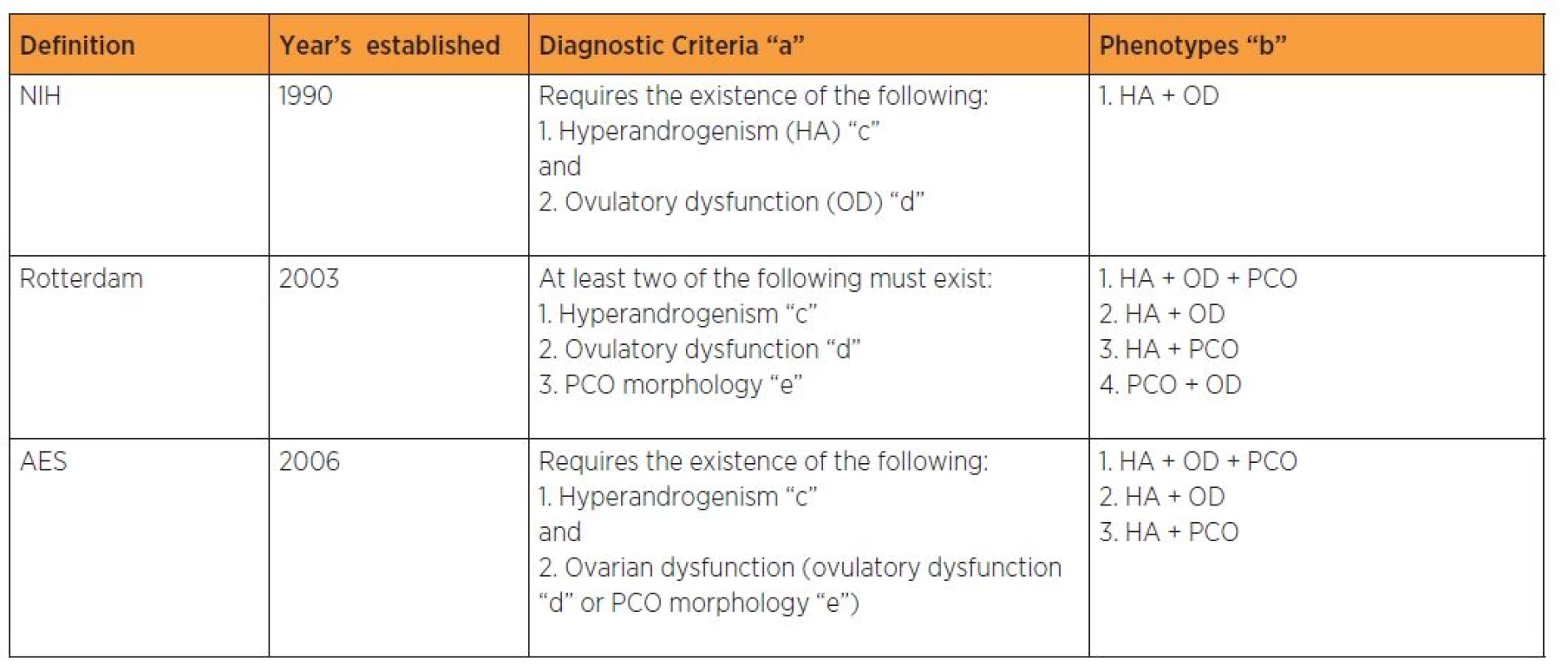
DIAGNOSTIC CRITERIA OF PCOS
There are three diagnostic criteria used to define PCOS as described in table 1. Previously the heterogeneity in the clinical signs of PCOS and the lack of uniformity in its defining symptoms had hampered its diagnosis, leaving millions of undiagnosed women without treatment. In 1990, the National Institute of Health (NIH) established that a diagnosis of PCOS required the simultaneous presence of oligomenorrhoea/anovulation and clinical or biochemical hyperandrogenism, after all other possible etiologies had been excluded. Later on in 2003, a consensus was reached among specialist in Rotterdam, about a comprehensive definition of PCOS. At least two of the following three criteria of oligomenorrhoea/ anovulation, clinical or biochemical hyperandrogenism, and polycystic ovaries on ultrasound should be present when other causes of those clinical features have been excluded [41].The Androgen Excess-PCOS Society (AE-PCOS) criteria which emerged in 2006 included women with oligomenorrhoea/ oligoovulation or ultrasonographically detected polycystic ovaries, and, clinical or biochemical hyperandrogenism after the exclusion of all other possible etiologies. The criterion concerning ovarian morphology was added to the initial diagnostic criteria; however, it is absent in 5–20% of cases [19, 57]. The inclusion of phenotypes unrelated to hyperinsulinism remains under debate. Some authors argue that PCOS is a primarily hyperandrogenic condition, therefore phenotypes that do not include this feature, should not be accepted as such [7].
THE PREVALENCE OF PCOS
The prevalence of PCOS critically depends on ethnic origin, race, phenotype, environmental factors, possible interaction between environmental and genetic factors (represented by race) as well as the criteria used to define PCOS [53]. Other factors that may influence the prevalence of PCOS include the type of study, methodology, and the age of the group recruited in the study. The majority of previous studies have defined PCOS by relying on a restricted number of features, mainly morphological confirmation of polycystic ovaries. The prevalence of PCOS has increased with the use of different diagnostic criteria, but this increase in the prevalence could also be due to an increase in education and socio-economic related factors which had a positive reflection on women‘s awareness. Thus, more undiagnosed women with PCOS have been diagnosed. Furthermore, the prevalence of PCOS also increases with age progression. In 2010, the World Health Organization (WHO) estimated that PCOS affects 3.4% of women worldwide [91]. Compared to younger reproductive women, older women have significantly higher concentrations of FSH and oestradiol and significantly lower concentrations of LH, progesterone, Inhibin A, and Inhibin B hormones [93].
When the definition of PCOS relies exclusively on the appearance of polycystic ovaries at either sonography or surgery, the prevalence of PCOS is anything between 1-20% of randomly targeted women [20, 36, 75]. However, since up to 25% of patients with this ovarian morphology on sonography are asymptomatic, the previous definition is considered to be nonspecific [86]. Furthermore, many patients with hyperandrogenic oligoovulation do not exhibit polycystic - appearing ovaries [32, 70, 75, 86]. Later on in 1990,an agreement was reached with regard to the definition of PCOS during the NIH/NICHHD conference. The majority of conference attendees believed that PCOS should be defined by 1. ovulatory dysfunction, 2. clinical evidence of hyperandrogenism (hirsutism, acne or androgenic alopecia) and/or hyperandrogenaemia, and 3. exclusion of related disorders such as hyperprolactinaemia, thyroid disorders, and non-classic adrenal hyperplasia (NCAH) [95].
Studies performed in the USA revealed a prevalence rate of 5–10%. These studies consistently report that the PCOS are more common in Mexican - American than White or African-American women. Studies conducted in European countries reported a prevalence of 4.6 to 8% [5, 8, 27, 56, 60, 65]. Besides ethnicity, other studies have shown that the prevalence of PCOS varies depending on the diagnostic criteria used [62, 64, 94]. Thus, the estimated prevalence using the Rotterd-am criteria is two to three times greater than those obtained using the NIH/NICHD criteria [62, 64, 94], as shown in figure 1.

The prevalence rates are higher in those with gestational diabetes, those who have premature adrenarche and in those with first-degree relatives who have PCOS [45, 47, 51]. Unfortunately, data regarding PCOS prevalence in most developing countries are scarce. A Chinese study used the NIH criteria to evaluate 915 women attending a healthcare unit has reported a prevalence of 2.2% [19]. A population-based survey preformed in Sri Lanka involving 2915 women reported a prevalence of 6.3% when the Rotterdam criteria were used [57]. The prevalence of PCOS was 6% in a Mexican study performed in Mexico City which evaluated 150 female volunteers [67]. A possible interaction between genetics and environmental factors may occur following immigration. This presumption is based on a result obtained from a study performed in the USA which evaluated Mexican-American women. It was found that the prevalence of PCOS in this group is 12.6 % [40].
METABOLIC SYNDROME AND PCOS
Metabolic syndrome is a constellation of metabolic disorders that directly increase the risk of coronary heart disease (CHD), CVD and DMT2. According to a definition released by the World Health Organization (WHO) in 1999 metabolic syndrome can be defined by the following criteria: having insulin resistance, glucose intolerance, impaired glucose tolerance or DMT2in combination with at least two of the parameters including hypertension(≥140/90 mm Hg), hypertriglyceridaemia (≥150 mg/dl) and/ or low HDL-cholesterol (<35 mg/dl in men and <39 mg/dl in women), obesity as measured by waist/hip ratio (>0.9 in men and >0.85 in women) and/or body mass index (BMI) (>30 kg/m2) and microalbuminuria (urinary albumin excretion rate ≥20 µgm/minute or albumin/ creatine ratio ≥30 µgm/mg) [97]. Subsequently, more definitions have been released, and in order to reach a consensus regarding a precise definition of metabolic syndrome, the International Diabetes Federation (IDF) defined metabolic syndrome as central obesity (based on race - and gender-specific waist circumference (WC) cutoffs) plus any two of the following: raised triglycerides of ≥150 mg/dl (1.7 mmol/l) or history of specific treatment for this lipid abnormality, reduced HDL cholesterol of< 40 mg/dl (1.03 mmol/l) in males and <50 mg/dl (1.29 mmol/l) in females or a history of specific treatment for this lipid abnormality, raised systolic blood pressure(BP) of ≥130 mm Hg or diastolic BP of ?85 mm Hg or on treatment for previously diagnosed hypertension, or, a raised Fasting Plasma Glucose (FPG) of ≥100 mg/dl (5.6 mml/l) or previously diagnosed DMT2 [88]. Nevertheless, experts worldwide preferred using the National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III) definition because of its simplicity and applicability in clinical practice and research. This definition identifies abdominal obesity (represented by WC) as the main factor and BMI is no longer considered. Besides abdominal obesity, the other parameters included in this definition area WCof ≥102 cm in men and ≥88 cm in women, hypertriglyceridaemia of ≥150 mg/dl (1.695 mmol/l), low HDL-C of <40 mg/dl in men and <50 mg/dl in women, an elevated blood pressure (BP) of >130/85 mm Hg and a high fasting glucose of >110 mg/dl.
Metabolic Syndrome and PCOS are both very common and inter-related conditions that many women experience. The mechanism of developing metabolic syndrome shares many similarities with the mechanism of PCOS development. Environmental factors, genetic predisposition, behavioral factors and the possible interaction between these factors are believed to be the main causes of both metabolic syndrome and PCOS. Furthermore, related diseases such as DMT2 and CVD in both circumstances develop through similar mechanisms. The existence of insulin resistance in both conditions increases the chance of hyperinsulinaemia and promotes further weight gain creating a vicious cycle. In addition, this situation makes it even more difficult to differentiate between the causes of some metabolic disorders developed later on in life. Obesity, in particular abdominal obesity, hyperinsulinaemia, dyslipidaemia, and glucose intolerance are present in women with PCOS as well as women with metabolic syndrome [30].
There is a large consistency among studies showing that the prevalence of metabolic syndrome is significantly higher in patients with PCOS compared to the general population regardless of ethnicity and the applied metabolic syndrome definition [15, 22, 28, 43, 48]. In a large cross-sectional study by Panidis and colleaguesinvolving 1223 patients with PCOS, the prevalence of metabolic syndrome was higher (P = 0.021) in women with PCOS (15.8%) than in controls (10.1%), when the National Cholesterol Education Program Adult Treatment Panel III definition of Metabolic syndrome was used. This result did not apply when using the three more recent metabolic syndrome definitions [71]. In another study the risk of metabolic syndrome is 11 fold higher in women with PCOS compared to age-matched controls [28].
METABOLIC SYNDROMEAND METABOLIC DISORDERS
Women with metabolic syndrome are at increased risk of developing metabolic disorders compared to the general population. There is abundant literature identifying insulin resistance, hyperinsulinaemia, obesity, dyslipidaemia, glucose intolerance, and hypertension as risk factors for DMT2, CVD and atherosclerosis [31, 96, 98], as well as associating it with an increased risk of developing different types of gynaecological cancer [10, 12, 34, 69]. Figure 2 describes the contribution of insulin resistance and hyperinsulinaemia in the development of metabolic disorders.

TYPE 2 DIABETES (DMT2)
Insulin resistance is the main abnormality in DMT2 and is considered to be a strong predictor of the disease [25, 26]. The United Kingdom Prospective Diabetes Study found that 40% of diabetic women are obese [89]. Obesity is associated with an increase adipocyte size and number. Increased fat deposition is associated with an increased size of mature adipocytes and resistance to the antilipolytic effects of insulin, leading to elevated levels of plasma free fatty acids. Chronically increased plasma free fatty acids stimulate gluconeogenesis causing hepatic and muscle insulin resistance. An increased number of mature adipocytes also reduce the capacity of adipose tissue to store fat leading to diversion off at to unusual places such as muscle, liver and the abdominal cavity. Studies repeatedly reported that patients with insulin resistance, impaired glucose tolerance and DMT2 have increased triglyceride levels and elevated total cholesterol concentrations, with increased concentrations of small dense LDL and decreased concentrations of HDL [42]. Furthermore, patients with DMT2 have an increased risk of endothelial dysfunction and thrombosis [42].
CARDIOVASCULAR DISEASE (CVD)
Abdominal obesity in particular is associated with an increased risk of CVD morbidity and mortality [84, 85]. The contribution of body fat to the progression of atherosclerosis is potentially the most likely pathway to explain the relationship with CVD. Abdominal obesity or visceral fat is positively associated with insulin resistance, DMT2 and cardiovascular risk. Indeed lean patients with visceral fat, also known as metabolically obese normal-weight individuals are at increased risk of developing hypertriglycerideamia, hyperinsuli-naemia, DMT2, and cardiovascular diseases [80]. An unfavorable relationship with weight gain tended to be more pronounced in lean compared to obese individuals and in men compared to women [77]. However, in both, general obesity and abdominal adiposity are associated with the risk of death [74]. Mitchell and colleagues have suggested that genes that confer a risk of diabetes, and to a lesser extent hypertension, adversely alter the cardiovascular risk profile long before the manifestation of clinical disease [66].
CARDIOVASCULAR DISEASES AND TYPE 2 DIABETES
Generally, CVD is considered to be the end point of DMT2. According to the World Health Organization Multinational Study of Vascular Disease in Diabetes, CVD is considered to be the most common cause of death in diabetic patients, accounting for about 52% of the cause of mortality [68]. Compared to controls, patients with diabetes have two to four fold increases in CVD prevalence [55]. This is because diabetes is associated with abnormalities in numerous metabolic pathways, including lipid metabolism, fibrinolysis, coagulation and platelet function. Furthermore, patients with DMT2 and CVD share common genetic and environmental antecedents. Patients with DMT2 usually present with abnormal fat distribution, atherogenic lipid profiles and insulin resistance, all of which are considered to be risk factors for CVD [42]. The other factor that connects diabetes with CVD is hypertension. More than one half of diabetic women are hypertensive or receiving hypertensive therapy [89].
Patients with hypertension more commonly present with other risk factors for CVD, like insulin resistance, obesity, diabetes or dyslipidaemia besides target organ damage. As these risk factors most probably interact, the overall risk of hypertension is increased, although blood pressure elevation is only mild or moderate. Individuals with overt diabetes (whether Type 1 or 2) often become hypertensive soon after developing renal damage. If such complications are not treated hypertensive nephrosclerosis will develop causing a vicious cycle between kidney damage and heart damage, and, if no action is taken, heart failure will ensue. Thus, an increase in the prevalence of diabetes causes a steep increase in CVD patients [97]. Consequently, an increase in the prevalence of diabetes in patients with PCOS is expected to lead to a significant increase in the prevalence of CVD in these patients.
GYNAECOLOGICAL CANCER
Among gynaecological cancer, an increased the risk of endometrial cancer was found to be associated with the different features of metabolic syndrome [12, 34, 78]. The multivariate odd ratios of endometrial cancer were 2.18 for DMT2, 1.77 for hypertension, 1.20 for hyperlipidemia, between 1.62 and 2.23 for various definitions of central obesity and 3.83 for women with a body mass index (BMI) >30 kg/m2. The risk of endometrial cancer was significantly increased for women with metabolic syndrome, the odd ratios ranging between 1.67 and 2.77 when waist circumference was included in the metabolic syndrome definition and 8.40 when BMI was considered instead [78]. Similarly in a large retrospective cohort where 290,000 women from Austria, Norway, and Sweden were enrolled during the period of 1974 to 2005, the relative risk of endometrial cancer in patients with metabolic syndrome was 1.37 (95% confidence interval: 1.28 to 1.46). The relative risk for women with metabolic syndrome and with high BMI was decreased before the age of 50 years, while menopausal women with metabolic syndrome in the cohort witnessed a significant increase in breast cancer death over the age of 60 years [10, 12]. However, the relationship between the presence of metabolic syndrome and breast cancer risk were inconsistent [1, 50, 81]. No relationship was reported in the same cohort between the presence of metabolic syndrome and ovarian cancer [11]. In a systematic review relying on a meta-analysis that included 38,940 cases of cancer from 116 datasets belong to 43 studies, Esposito and colleagues [34] found that the presence of metabolic syndrome was associated with endometrial cancer (1.61, P = 0.001) and breast cancer in postmenopausal women (1.56,P = 0.017). The risk factors associated with metabolic syndrome may confound the effect of PCOS, and consequently this may cause overestimation of the role of PCOS in the development of metabolic disorders.
GYNAECOLOGICAL CANCER AND TYPE 2 DIABETES
Diabetes is associated with an increased risk of cancer in general. However, the mechanism/s connecting cancer and diabetes are (with rare exceptions) incompletely understood [39]. On the other hand, hyperinsulinaemia (which is considered to be the main feature in patients with DMT2) causes a reduction in the hepatic synthesis and blood levels of sex hormone binding globulin, leading to increases in bioavailable oestrogen and increased levels of bioavailable (free) testosterone in women [14]. Hyperinsulinaemia is also associated with increased androgen synthesis in the ovaries. Elevated endogenous sex steroid levels are associated with a higher risk of postmenopausal breast and gynaecological cancer [14]. Hyperinsulinaemia and hyperandrogenaemia are both main features of patients with PCOS and may play a pivotal role in the pathogenesis of PCOS (figure 2).
Regardless of the existence of PCOS, diabetic women have two to three fold increases in the risk of developing endometrial cancer [38, 58]. The risk increases up to 6 fold if diabetic women are obese, and jump up to 10 fold if diabetic obese women do not exercise [80]. Compared to controls, the covariate adjusted hazard ratio for breast and endometrial cancer was 1.42 (95% confidence interval (CI): 1.34–1.50) and 1.71 (95% CI: 1.48–1.97), respectively in women with diabetes. The highest increase in risk of breast cancer was observed in diabetic women aged >65 years, while the highest increase in risk of endometrial cancer was observed in diabetic women aged ≤50 years [17]. Several other studies have shown a modest increase in the incidence of breast cancer in newly diagnosed patients with DMT2 [13, 44, 59]. Finally, the relationship between DMT2 and ovarian cancer is vague. A recent large-scale population based cohort study provided little support on the alleged association between DMT2 and the risk of ovarian cancer. The study reported an incidence rate of 1.87 for patients with ovarian cancer compared to 1.79 for controls [16].
GYNAECOLOGICAL CANCER AND CARDIOVASCULAR DISEASES
Although no direct relationship has yet been reported between breast or gynecological cancer and CVD, they share similar risk factors. These include non-modifiable risk factors such as age and modifiable risk factors such as a BMI of >25, android obesity, a high fat diet, physical inactivity, smoking, alcohol consumption and air pollution [52]. Furthermore, the direct effects of cardio-toxic cancer therapies (namely radiation or chemotherapy) and the interaction between specific lifestyle patterns (initiated after treatment or which existed before) and weight gain (which is usually associated with physical inactivity) will exacerbate the risk of CVD [92]. Thus, women diagnosed with cancer usually die of CVD rather than progression or recurrence of their cancer. In a large study carried out in the USA [73] which involved 63, 566 women diagnosed with breast cancer, cardiovascular disease was the primary cause of death in the study population (15.9%; 95% CI: 15.6–16.2), followed closely by breast cancer (15.1%; 95% CI: 14.8–15.4).
A positive relationship between breast cancer radiotherapy (in particular left breast cancer) and cardiovascular disease has been reported [23]. The radiation-related increase in the risk of major coronary events began within the first 5 years after exposure [24], while others reported early increase in risk of coronary events in patients with Hodgkin‘s lymphoma who received radiotherapy [2, 87]. However, the effect of preexisting cardiac risk factors on the risk of radiation-related ischemic heart disease has never been targeted [23].
METABOLIC SYNDROME AND INFERTILITY
Infertility is the inability to achieve pregnancy after one year of regular, unprotected intercourse. A specific metabolic syndrome features such as visceral fat and insulin resistance are believed to be the primary cause of PCOS. Irregular ovulation, or lack of ovulation in patients with PCOS, making PCOS the most common cause of woman infertility. Roughly, around 30% of infertility is due to PCOS [21]. In addition, a recent study reported that 72% of PCOS women suffer from infertility [49]. Experiencing difficulties in becoming pregnant could be the main reason leading to the detection of PCOS. Infertility in most patients with PCOS results from the absence of ovulation (anovulation); nevertheless anovulation may not be the only reason for infertility. Other reasons of infertility may include decreased or diminished ovarian reserve, functional hypothalamic amenorrhoea, premature ovarian insufficiency, tubal patency, endometriosis, pelvic or adnexal adhesions and uterine abnormalities.
Infertile women with PCOS, mainly those aged ≥25 years or with central obesity (a waist hip ratio of ≥0.85), are at a higher risk of developing metabolic syndrome [61]. Furthermore, having a large amount of visceral fat also increased the chance of developing PCOS. Visceral fat is associated with insulin resistance and hyperinsulinaemia [79]. An increased insulin level (hyperinsulinaemia) is positively associated with hyperandrogenism which considered to be a key feature in patients with PCOS. Thus, both insulin resistance and excess androgen levels are associated with visceral fat (waist circumference). However, insulin resistance/sensitivity varies according to the menstrual pattern. As a result, women with regular cycles have less insulin resistance compared to women with irregular cycles, irrespective of their BMI.
Lifestyle changes, such as weight loss and exercise can trigger body changes and restore the menses in women with PCOS (figure 3). This is because such changes in lifestyle can cause significant reduction in circulating insulin levels and decrease luteinizing hormone levels, which are considered to be the basis of therapeutic principles. Indeed exercise with or without weight loss is associated with substantial reductions in total and abdominal fat [79]. The majority of patients with PCOS are overweight or obese, or have a normal body mass indices (BMI) but are metabolically obese with significant amounts of visceral fat. Visceral fat represented by waist circumference correlates positively with insulin resistance and metabolic imbalance. Thus the larger the visceral fat depot, the worse is the metabolic imbalance. This problem can be elucidated by weight loss, as 10% weight loss reduces 30% of visceral fat [96]. As the majority of patients with PCOS are insulin resistant and reversing this situation will cause an improvement in insulin action. Furthermore, weight reduction causes a significant improvement in endocrine and ovarian function in patients with PCOS [63, 90].

CONCLUSION
The prevalence of metabolic syndrome is significantly higher in patients with PCOS compared to the general population regardless of ethnicity or the applied metabolic syndrome definition. Supportive evidence shows that insulin resistance, hyperinsulinaemia, obesity, dyslipidaemia, glucose intolerance and hypertension are more common in patients with PCOS.This increase in the prevalence of metabolic syndrome places women with PCOS at a great risk of developing metabolic consequences such as DMT2, CVD and endometrial cancer. Infertility in women with PCOS is common and is positively correlated with metabolic syndrome. Infertility due to anovulation can be treated with significant modification in lifestyle, which may include weight loss and regular exercise.
Acknowledgment
I would like to thank Dr. Nawaal Davids fromthe Department of Chemical Pathology Tygerberg Hospital and Stellenbosch Medical School for reading this article.
Dr. Aus Tariq Ali
Department of Pathology
Tygerberg Hospital and Stellenbosch Medical School
Po Box 19113
Tygerberg 7505
Cape Town
South Africa
e-mail: atali@sun.ac.za
Sources
1. Agnoli, C., Berrino, F., Abagnato, CA., et al. Metabolic syndrome and postmenopausal breast cancer in the ORDET cohort: a nested case-control study. Nutr Metab Cardiovasc Dis, 2010, 20, p. 41–48.
2. Aleman, BM., van den Belt-Dusebout, AW., Klokman, WJ., et al. Long-term causes pecific mortality of patients treated for Hodgkin’s disease. J Clin Oncol, 2003, 21, p. 3431–3439.
3. Ali, AT., Ferris, WF., Naran, NH., Crowther, NJ. Insulin resistance in the control of body fat distribution: a new hypothesis. Horm Metab Res, 2011, 43(2), p. 77–80.
4. Alvarez Cosmea, A., Lopez Hernandez, V., Suarez Garcia, S., et al. Differences in the prevalence of metabolic syndrome according to the ATP-III and WHO definitions. Medical Clinics, 2005, 124, p. 368–370.
5. Asuncion, M., Calvo, RM., San Millan, JL., et al. A prospective study of the prevalence of the polycystic ovary syndrome in unselected Caucasian women from Spain. J Clin Endocrinol Metab, 2000, 85, p. 2434–2438.
6. Azevedo, MF., Costa, EC., Oliveira, AI., et al. Elevated blood pressure in women with polycystic ovary syndrome: prevalence and associated risk factors. Rev Bras Ginecol Obstet, 2011, 33(1), p. 31–36.
7. Azziz, R., Carmina, E., Dewailly, D., et al. Positions statement; criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: an Androgen Excess Society guideline. J Clin Endocrinol Metabol, 2006, 91(11), p. 4237–4245.
8. Azziz, R., Woods, KS., Reyna, R., et al. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab, 2004, 89, p. 2745–2749.
9. Barber, TM., McCarthy, MI., Wass, JA., Franks, S. Obesity and polycystic ovary syndrome. Clin Endocrinol (Oxf), 2006, 65, p. 137–145.
10. Bjørge, T., Lukanova, A., Jonsson, H., et al. Metabolic syndrome and breast cancer in the Me-Can (Metabolic Syndrome and Cancer) project. Cancer Epidemiol Biomarkers Prev 2010, 19, p. 1737–1745.
11. Bjørge, T., Lukanova, A., Tretli, S., et al. Metabolic risk factors and ovarian cancer in the Metabolic Syndrome and Cancer project. Int J Epidemiol, 2011, 40(6), p. 1667–1677.
12. Bjørge, T., Stocks, T., Lukanova, A., et al. Metabolic syndrome and endometrial carcinoma. Am J Epidemiol, 2010, 171, p. 892–902.
13. Boyle, P., Boniol, M., Koechlin, A., et al. Diabetes and breast cancer risk: a meta-analysis Brit J Cancer, 2012, 107, p. 1608–1617.
14. Calle, EE., Kaaks, R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer, 2004, 4(8), p. 579–591.
15. Carmina, E., Napoli, N., Longo, RA., et al. Metabolic syndrome in polycystic ovary syndrome (PCOS): lower prevalence in southern Italy than in the USA and the influence of criteria for the diagnosis of PCOS. Eur J of Endocrinol, 2006, 154, p. 141–145.
16. Chen, HF., Chang, YH., Ko, MC., Li, CY. A large scale population-based cohort study on the risk of ovarian neoplasm in patients with type 2 diabetes mellitus. Gynecol Oncol, 2014, 134(3), p. 576–580.
17. Chen, HF., Liu, MD., Chen, P., et al. Risks of Breast and Endometrial Cancer in Women with Diabetes: A Population-Based Cohort Study. PLoS One, 2013, 8(6), e67420.
18. Chen, X., Yang, D., Li, L., et al. Abnormal glucose tolerance in Chinese women with polycystic ovary syndrome. Hum Reprod, 2006, 21, p. 2027–2032.
19. Chen, X., Yang, D., Mo, Y., et al. Prevalence of polycystic ovary syndrome in unselected women from southern China. Eur J Obstet Gynecol Reprod Biol, 2008, 139(1), p. 59–64.
20. Clayton, RN., Ogden, V., Hodgkinson, J., et al. How common are polycystic ovaries in normal women and what is their significance for the fertility of the population. Clin Endocrinol (Oxf), 1992, 37, p. 127–134.
21. Clayton, RN., Ogden, V., Hodgkinsonc, J. How common are polycystic ovaries in normal women and what is their significance for the fertility of populations. Clin Endocrinol, 1992, 39, p. 623–632.
22. Cussons, AJ., Watts, GF., Burke, V., et al. Cardiometabolic risk in polycystic ovary syndrome: a comparison of different approaches to defining the metabolic syndrome. Human Reprod, 2008, 23, p. 2352–2358.
23. Darby, SC., Ewertz, M., Hall, P. Ischemic heart disease after breast cancer radiotherapy. N Engl J Med, 2013, 368(26), p. 2527.
24. Darby, SC., Ewertz, M., McGale, P., et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med, 2013, 368(11), p. 987–998.
25. DeFronzo, RA. Pathogenesis of type 2 diabetes mellitus. Med Clin North Am, 2004, 88(4), p. 787–835.
26. DeFronzo, RA., Tripathy, D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care, 2009, 32, Suppl. 2, p. S157–163.
27. Diamanti-Kandarakis, E., Kouli, CR., Bergiele, AT., et al. A survey of the polycystic ovary syndrome in the Greek island of Lesbos: hormonal and metabolic profile. J Clin Endocrinol Metab, 1999, 84(11), p. 4006–4011.
28. Dokras, A., Bochner, M., Hollinrake, E., et al. Screening women with polycystic ovary syndrome for metabolic syndrome. Obstet Gynecol, 2005, 106, p. 131–137.
29. Dunaif, A. Insulin resistance and the polycystic ovary syndrome: mechanism and implications for pathogenesis. Endocr Rev, 1997, 18(6), p. 774–800.
30. Ehrmann, DA., Barnes, RB., Rosenfield, RL., et al. Prevalence of impaired glucose tolerance and diabetes in women with polycystic ovary syndrome. Diabetes Care, 1999, 22, p. 141–146.
31. Eeg-Olofsson, K., Gudbjörnsdottir, S., Eliasson, B., et al., on behalf of the NDR. The triglycerides-to-HDL-cholesterol ratio and cardiovascular disease risk in obese patients with type 2 diabetes: An observational study from the Swedish National Diabetes Register (NDR). Diabetes Res Clin Pract, 2014.
32. elTabbakh, GH., Lotfy, I., Azab, I., et al. Correlation of the ultrasonic appearance of the ovaries in polycystic ovarian disease and the clinical, hormonal, and laparoscopic findings. Am J Obstet Gynecol, 1986 ,154, p. 892–895.
33. Escobar-Morreale, HF., San Millan, JL. Abdominal adiposity and the polycystic ovary syndrome. Trends Endocrinol Metab, 2007, 18(7), p. 266–272.
34. Esposito, K., Chiodini, P., Colao, A., et al. Metabolic syndrome and risk of cancer: a systematic review and meta-analysis. Diabetes Care, 2012, 35(11), p. 2402–2411.
35. Farquhar, C. Introduction and history of polycystic ovary syndrome. In: Kovacs. G., Norman. R., editors. Polycystic Ovary Syndrome. 2nd ed. Cambridge, UK: Cambridge University Press, 2007, p. 4–24.
36. Farquhar, CM., Birdsall, M., Manning, P., et al. The prevalence of polycystic ovaries on ultrasound scanning in a population of randomly selected women. Aust NZ Obstet Gynaecol, 1994, 34, p. 67–72.
37. Ford, ES., Giles, WH., Dietz, WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. J Amer Med Assoc, 2002, 287, p. 356–359.
38. Friberg, E., Mantzoros, CS., Wolk, A. Diabetes and risk of endometrial cancer: a population-based prospective cohort study. Cancer Epidemiol Biomarkers Prev, 2007, 16, p. 276–280.
39. Giovannucci, E., Harlan, DM., Archer, MC., et al. Diabetes and cancer: a consensus report. Diabetes Care, 2010, 33(7), p. 1674–1685.
40. Goodarzi, MO., Quinones, MJ., Azziz, R.,et al. Polycystic ovary syndrome in Mexican-Americans: prevalence and association with the severity of insulin resistance. Fertil Steril, 2005, 84(3), p. 766–769.
41. Group PCW: Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril, 2004, 81(1), p. 19–25.
42. Haffner, SM., Stern, MP., Hazuda, HP., et al. Cardiovascular risk factors in confirmed prediabetic individuals. JAMA, 1990, 263, p. 2893–2898.
43. Hahn, S., Tan, S., Sack, S., et al. Prevalence of the metabolic syndrome in German women with polycystic ovary syndrome. Exp Clin Endocrinol Diabetes, 2007, 115, p. 130–135.
44. Hardefeldt, PJ., Edirimanne, S., Eslick, GD. Diabetes increases the risk of breast cancer: a meta-analysis. Endocr Relat Cancer, 2012, 19, 19(6), p. 793–803.
45. Holte, J., Bergh, T., Berne, C., et al. Restored insulin sensitivity but persistently increased early insulin secretion after weight loss in obese women with polycystic ovary syndrome. J Clin Endocrinol Metab, 1995, 80, p. 2586–2593.
46. Hopkinson, ZE., Sattar, N., Fleming, R., Greer, IA. Polycystic ovarian syndrome: the metabolic syndrome comes to gynaecology. BMJ, 1998, 317(7154), p. 329–332.
47. Ibanez, L., Dimartino-Nardi, J., Potau, N. Premature adrenarche – normal variant or forerunner of adult disease? Endocr Rev, 2000, 21, p. 671–696.
48. Indhavivadhana, S., Wongwananuruk, T., Rattana-chaiyanont, M., et al. Prevalence of metabolic syndrome in reproductive-aged polycystic ovary syndrome Thai women. J Med Assoc Thai, 2010, 93(6), p. 653–660.
49. Joham, AE., Teede, HJ., Ranasinha, S., et al. Prevalence of infertility and use of fertility treatment in women with polycystic ovary syndrome: data from a large community-based cohort study. J Womens Health (Larchmt), 2015, 24(4), p. 299–307.
50. Kabat, GC., Kim, M., Chlebowski, RT., et al. A longitudinal study of the metabolic syndrome and risk of postmenopausal breast cancer. Cancer Epidemiol Biomarkers Prev, 2009, 18, p. 2046–2053.
51. Kahsar-Miller, M., Nixon, C., Boots, LR., et al. Prevalence of the polycystic ovary syndrome (PCOS) among first degree relatives of patients with PCOS. Fertil Steril, 2001, 75, p. 53–58.
52. Katsoulis, M., Dimakopoulou, K., Pedeli, X., et al. Long-term exposure to traffic-related air pollution and cardiovascular health in a Greek cohort study. Sci Total Environ, 2014, 490, p. 934–940.
53. Kauffman, RP., Baker, VM., Dimarino, P., et al. Polycystic ovarian syndrome and insulin resistance in white and Mexican American women: a comparison of two distinct populations. Am J ObstetGynecol, 2002, 187(5), p. 1362–1369.
54. Kaur, JA. Comprehensive review on metabolic syndrome. Cardiol Res Pract, 2014, 2014, p. 943162.
55. Kirpichnikov, D., Sowers, J. Diabetes mellitus and diabetes-associated vascular disease. Trends Endocrinol Metab, 2001, 12, p. 225–229.
56. Knochenhauer, ES., Key, TJ., Kahsar-Miller, M., et al. Prevalence of the polycystic ovary syndrome in unselected black and white women of the southeastern United States: a prospective study. J Clin Endocrinol Metab, 1998, 83(9), p. 3078–3082.
57. Kumarapeli, V., Seneviratne, de A., Wijeyaratne, CN., et al. A simple screening approach for assessing community prevalence and phenotype of polycystic ovary syndrome in a semi-urban population in Sri Lanka. Am J Epidemiol, 2008, 168(3), p. 321–328.
58. La Vecchia, C., Negri, E., Franceschi, S., et al. A case-control study of diabetes mellitus and cancer risk. Br J Cancer, 1994, 70, p. 950–953.
59. Liao, S., Li, J., Wei, W., et al. Association between diabetes mellitus and breast cancer risk: a meta-analysis of the literature. Asian Pac J Cancer Prev, 2011, 12, p. 1061–1065.
60. Lindholm, A., Andersson, L., Eliasson, M., et al. Prevalence of symptoms associated with polycystic ovary syndrome. Int J Gynaecol Obstet, 2008, 102(1), p. 39–43.
61. Mandrelle, K., Kamath, MS., Bondu, DJ., et al. Prevalence of metabolic syndrome in women with polycystic ovary syndrome attending an infertility clinic in a tertiary care hospital in south India. J Hum Reprod Sci, 2012, 5(1), p. 26–31.
62. March, WA., Moore, VM., Willson, KJ., et al. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod, 2010, 25(2), p. 544–551.
63. Marsh, KA., Steinbeck, KS., Atkinson, FS., et al. Effect of a low glycemic index compared with a conventional healthy diet on polycystic ovary syndrome. Am J Clin Nutr, 2010, 92, p. 83–92.
64. Mehrabian, F., Khani, B., Kelishadi, R., Ghanbari, E. The prevalence of polycystic ovary syndrome in Iranian women based on different diagnostic criteria. Endokrynol Pol, 2011, 62(3), p. 238–242.
65. Michelmore, KF., Balen, AH., Dunger, DB., Vessey, MP. Polycystic ovaries and associated clinical and biochemical features in young women. Clin Endocrinol (Oxf), 1999, 51, p. 779–786.
66. Mitchell, BD., Almasy, LA., Rainwater, DL.,et al. Diabetes and hypertension in Mexican American families: relation to cardiovascular risk. Am J Epidemiol, 1999, 149(11), p. 1047–1056.
67. Moran, LJ., Misso, ML., Wild, RA., Norman, RJ. Impaired glucose tolerance, type 2 diabetes and metabolic syndrome: a systematic review and meta-analysis. Hum Reprod Update, 2010, 16(4), p. 347–363.
68. Morrish, NJ., Wang, SL., Stevens, LK., et al. Mortality and causes of death in the WHO Multinational Study of Vascular Disease in Diabetes. Diabetologia, 2001, 44, Suppl 2, p. S14–21.
69. Nagel, G., Concin, H., Bjorge, T., et al. Metabolic syndrome and rare gynecological cancers in the metabolic syndrome and cancer project (Me-Can). Ann Oncol, 2011, 22(6), p. 1339–1345.
70. Orsini, LF., Venturoli, S., Lorusso, R., et al. Ultrasonic findings in polycystic ovarian disease. Fertil Steril, 1985, 43, p. 709–714.
71. Panidis, D., Macut, D., Tziomalos, K., et al. Prevalence of metabolic syndrome in women with polycystic ovary syndrome. Clin Endocrinol (Oxf), 2013, 78(4), p. 586–592.
72. Park, YW., Zhu, S., Palaniappan, L., et al. The metabolic syndrome: prevalence and associated risk factor findings in the US population from the third National Health and Nutrition Examination Survey, 1988–1994. Arch Internal Med, 2003, 163, p. 427–436.
73. Patnaik, JL., Byers, T., DiGuiseppi, C., et al. Cardiovascular disease competes with breast cancer as the leading cause ofdeath for older females diagnosed with breast cancer: a retrospective cohort study. Breast Cancer Res, 2011, 13(3), p. R64.
74. Pischon, T., Boeing, H., Hoffmann, K., et al. General and abdominal adiposity and risk of death in Europe. N Engl J Med, 2008, 359(20), p. 2105–2120.
75. Polson, DW., Wadsworth, J., Adams, J., Franks, S. Polycystic ovaries – a common finding in normal women. Lancet, 1988, 1, p. 870–872.
76. Prasad, H., Ryan, DA., Celzo, MF., Stapleton, D. Metabolic syndrome: definition and therapeutic implications. Postgrad Med, 2012, 124(1), p. 21–30.
77. Rainwater, DL., Mitchell, BD., Comuzzie, AG., et al. Association among 5-year changes in weight, physical activity, and cardiovascular disease risk factors in Mexican Americans. Am J Epidemiol, 2000, 152, p. 974–982.
78. Rosato, V., Zucchetto, A., Bosetti, C., et al. Metabolic syndrome and endometrial cancer risk. Ann Oncol, 2011, 22 (4), p. 884–889.
79. Ross, R., Janssen, I., Dawson, J., et al. Exercise-induced reduction in obesity and insulin resistance in women: a randomized controlled trial. Obes Res, 2004, 12(5), p. 789–798.
80. Ruderman, N., Chisholm, D., Pi-Sunyer, X., Schneider, S. The metabolically obese, normal-weight individual revisited. Diabetes, 1998, 47(5), p. 699–713.
81. Russo, A., Autelitano, M., Bisanti, L. Metabolic syndrome and cancer risk. Eur J Cancer, 2008, 44, p. 293–297.
82. Santos, AC., Lopes, C., Barros, H. Prevalence of metabolic syndrome in the city of Porto. Revista Portuguesa de Cardiologia (Lisboa), 2004, 23, p. 45–52.
83. Sartor, BM., Dickey, RP. Polycystic ovarian syndrome and the metabolic syndrome. Am J Med Sci, 2005, 330, p. 336–342.
84. Sjöström, LV. Morbidity of severely obese subjects. Am J Clin Nutr, 1992, 55 (Suppl. 2), p. 508S–515S.
85. Sjöström, LV. Mortality of severely obese subjects. Am J Clin Nutr, 1992, 55 (Suppl. 2), p. 516S–523S.
86. Swanson, M., Sauerbrei, EE., Cooperberg, PL. Medical implications of ultrasonically detected polycystic ovaries. J Clin Ultrasound, 1981, 9, p. 219.
87. Swerdlow, AJ., Higgins, CD., Smith, P., et al. Myocardial infarction mortality risk after treatment for Hodgkin disease: a collaborative British cohort study. J Natl Cancer Inst, 2007, 99, p. 206–214.
88. The IDF consensus worldwide definition of the metabolic syndrome. Available at: http://www.idf.org/webdata/docs/MetSyndrome_FINAL.pdf. (Last accessed on 2014 4thDec).
89. Turner, RC., Mann, J., Oakes, S., et al. United Kingdom Prospective Diabetes Study, a multicenter study. Hypertension, 1985, 7 (Suppl II), p. 8–13.
90. Vause, TD., Cheung, AP., Sierra, S., et al. Ovulation induction in polycystic ovary syndrome. J Obstet Gynaecol Can, 2010, 32(5), p. 495–502.
91. Vos, T., Flaxman, AD., Naghavi, M., et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet, 2012, 380 (9859), p. 2163–2196.
92. Weaver, KE., Foraker, RE., Alfano, CM., et al. Cardiovascular risk factors among long-term survivors of breast, prostate, colorectal, and gynecologic cancers: a gap in survivorship care?J Cancer Surviv, 2013, 7(2), p. 253–261.
93. Welt, CK., Adams, JM., Sluss, PM., Hall, JE. Inhibin A and inhibin B responses to gonadotropin withdrawal depends on stage of follicle development. J Clin Endocrinol Metab, 1999, 84, p. 2163–2169.
94. Yildiz, BO., Bozdag, G., Yapici, Z., et al. Prevalence, phenotype and cardiometabolic risk of polycystic ovary syndrome under different diagnostic criteria. Hum Reprod, 2012, 27(10), p. 3067–3073.
95. Zawadzki, JK., Dunaif, A. Diagnostic criteria for polycystic ovary syndrome: Towards a rational approach 1992. In Dunaif, A., Givens, JR., Haseltine, F., Merriam, GR., eds. Polycystic ovary syndrome. Boston: Blackwell, p. 377–384.
96. Zhang, PY. Cardiovascular disease in diabetes. Eur Rev Med Pharmacol Sci, 2014, 18(15), p. 2205–2214.
97. Zimmet, P., Alberti, KGMM., Shaw, J. Global and societal implications of the diabetes epidemic. Nature, 2001, 414, p. 782–787.
98. Zuliani, G., Morieri, ML., Volpato, S., et al. Insulin resistance and systemic inflammation, but not metabolic syndrome phenotype, predicts 9 years mortality in older adults. Atherosclerosis, 2014, 235(2), p. 538–545.
Labels
Paediatric gynaecology Gynaecology and obstetrics Reproduction medicineArticle was published in
Czech Gynaecology

2015 Issue 4
-
All articles in this issue
- Persistence in the treatment of overactive bladder (OAB) with Mirabegron in a multicentre clinical study
- Benefits and the accuracy of the intra-operative frozen section at suspected ovarian tumours
- The importance of HE4 in differential diagnosis of endometrial cancer
- Smoking in pregnancy – negative impacton mother and child Opinions on nicotine substitution
-
The issue of certain infectious diseases of pregnant women in everyday practice
Part II. Viral infections -
Surrogacy, yes or no?
Case report - Different outcome in two case reports of births showing symptoms of amniotic fluid embolism in the years 2002 and 2012 in the district hospital of Decin
- Polycystic ovary syndrome and metabolic syndrome
- Translation and cross-cultural adaptation of the Pregnancy Physical Activity Questionnaire (PPAQ) to the Brazilian population
- Czech Gynaecology
- Journal archive
- Current issue
- About the journal
Most read in this issue
- Polycystic ovary syndrome and metabolic syndrome
-
The issue of certain infectious diseases of pregnant women in everyday practice
Part II. Viral infections - Smoking in pregnancy – negative impacton mother and child Opinions on nicotine substitution
-
Surrogacy, yes or no?
Case report
