Hormonal contraception after intrahepatic cholestasis of pregnancy
Hormonálna antikoncepcia po prekonanej intrahepatálnej cholestáze gravidných
Cieľ štúdie:
Porovnanie užívania perorálnej hormonálnej antikoncepcie (HAK), nežiadúcich účinkov liečby a incidencie cholecystolitiázy u žien s anamnézou intrahepatálnej cholestázy gravidných (ICP) pred a po zavedení kyseliny ursodeoxycholovej (UDCA) do liečby.
Typ štúdie:
Epidemiologická regionálna.
Názov a sídlo pracoviska:
Gynekologicko-pôrodnícka klinika FN Trenčín.
Metodika:
Retrospektívna analýza 79 pôrodov s ICP v rokoch 1992–2004. Skupinu 1 tvorilo 36 žien, ktoré porodili v rokoch 1992–1996 a neboli liečené UDCA. Skupinu 2 tvorilo 43 žien, ktoré porodili v rokoch 1997–2004 a boli liečené UDCA v dávke 500–750 mg denne per os. V roku 2008 bol zaslaný dotazník liečeným ženám s ICP. V jednotlivých skupinách bolo analyzované užívanie HAK, prítomnosť žlčníkových kameňov, resp. odstránenia žlčníka a u užívateliek HAK incidencia ťažkostí porovnateľných s ICP.
Výsledky:
Frekvencia ICP bola rovnaká v oboch skupinách (0,4 % pôrodov). Návratnosť dotazníka bola 71 %. Analyzovaných bolo 56 žien s ICP – 26 v skupine 1 a 30 v skupine 2 (štatisticky nevýznamný rozdiel, p=0,81). Hormonálnu antikoncepciu v sledovanom súbore užívalo 15 žien (26,8 %) – 42,3 % v skupine 1 a 13,3 % v skupine 2 (štatisticky významný rozdiel, p=0,015). Pruritus počas užívania HAK udala len jedna žena v skupine 1. Frekvencia cholecystolitiázy alebo cholecystektómie bola 26,8 % v celom súbore – 38,5 % v skupine 1 a 16,7 % v skupine 2 (štatisticky významný rozdiel, p=0,043).
Záver:
Užívanie HAK po ICP je možné i na základe našich výsledkov považovať za bezpečné. Pri relatívne vysokom percente žien užívajúcich HAK sa zaznamenalo len minimum nežiadúcich účinkov.
Kľúčové slová:
hormonálna antikoncepcia, intrahepatálna cholestáza gravidných, pruritus, ursodeoxycholová kyselina, cholecystolitiáza.
Authors:
P. Kaščák 1
; M. Korbeľ 2
Authors‘ workplace:
Department of Obstetrics and Gynecology, General Hospital, Trenčín, Head of the department Peter Kaščák, MD, PhD.
1; 1st Department of Obstetrics and Gynecology University Hospital Bratislava, Head of the department Miroslav Borovský, Prof., MD, PhD.
2
Published in:
Ceska Gynekol 2011; 76(5): 374-378
Overview
Objective:
Comparing the use of oral contraception (OC), treatment side effects and the incidence of cholecystolithiasis in women with a history of intrahepatic cholestasis of pregnancy (ICP), before and after introducing ursodeoxycholic acid (UDCA) in the treatment.
Design:
Regional epidemiological.
Setting:
Department of Obstetrics and Gynecology, General Hospital, Trencin, Slovak Republic.
Methods:
Retrospective analysis of 79 deliveries with ICP between 1992 and 2004. Group 1 included 36 women who delivered between 1992 and 1996 and were not treated by UDCA. Group 2 included 43 women who delivered between 1997 and 2004 and were managed with a 500-750 mg daily dose of UDCA administered orally. In 2008, the questionnaire was sent to all treated women with ICP. The analysis was focused on OC use and presence of cholecystolithiasis, or cholecystectomy in individual groups. The incidence of difficulties comparable to ICP was analyzed in OC users.
Results:
The frequency of ICP was the same in both groups (0.4% of deliveries). The questionnaire response rate was 71%. Analysis was conducted in 56 women with ICP – in Groups 1 and 2 it was 26 and 30 women, respectively (the difference statistically insignificant, p=0.81). In the observed population, 15 women (26.8%) used hormonal contraception – in Groups 1 and 2 it was 42.3% and 13.3%, respectively (statistically significant difference, p=0.015). Only one woman in Group 1 reported pruritus during the use of OCs. The frequency of cholecystolithiasis or cholecystectomy occurrence was 26.8% in the entire population – in Groups 1 and 2 it was 38.5% and 16.7%, respectively (statistically significant difference, p=0.043).
Conclusion:
Based on our results it is possible to consider the use of OC in women with a history of ICP as safe. Only a minimum of side effects have been recorded in relatively high percentage of OC users.
Key words:
oral contraception, intrahepatic cholestasis of pregnancy, pruritus, ursodeoxycholic acid, cholecystolithiasis.
INTRODUCTION
Intrahepatic cholestasis of pregnancy (ICP) is clinically characterized by pruritus without evident skin lesions, with the usual onset in the third trimester. The onset in the second trimester of pregnancy is rare [13, 18]. ICP is generally considered a benign disease with a good prognosis for the mother [14, 20]. However, perinatal morbidity and mortality are significantly increased. Hepatobiliary diseases, such as non-alcoholic liver cirrhosis, cholecystitis and cholecystolithiasis, and non-alcoholic pancreatitis, constitute a delayed effect of ICP [21]. The most common delayed effect is the emergence of cholecystolithiasis, which occurs 2–3 times more often in the affected group of women than in the general population [13, 14, 20, 21]. Currently, ursodeoxycholic acid (UDCA) is the most effective treatment for ICP. UDCA normalizes, or decreases levels of liver enzymes, positively affects the patient’s subjective difficulties, and reduces the risk of fetal morbidity and mortality [1, 3, 4, 10]. Intrahepatic cholestatic jaundice was the first published adverse effect of oral contraceptives (OC) on the liver [19]. ICP and pill induced cholestasis (PIC) are closely linked, both clinically and epidemiologically. The latter is sometimes referred to as estrogen-induced cholestasis [24]. There might exist a genotypic correlation between both these disorders [17, 23]. The affected women used to experience significant difficulties at higher estrogen content in birth control pills. This fact has fueled a number of concerns expressed by female patients regarding the use of hormonal contraception, and a rather reluctant approach of the professional public to prescribe it due to the relative risk of contraindication [5, 15]. Guidance on the United Kingdom (UK) medical eligibility criteria for contraceptive use states that if the patient has a history of ICP, a combined oral contraceptive (COC) may be indicated as the benefit usually exceeds the risk [9]. It needs to be stressed, however, that the tendency of cholestasis to recur is higher when taking COCs. Women with a history of ICP should use contraceptives with low-dose estrogen or progesterone-only products [14]. The National Health System recommends that the patients with a history of ICP avoid oral contraceptives as there is a risk of developing similar difficulties (www.perinatal.org.uk/reviews). Also, the European Association for the Study of the Liver suggests that similar difficulties might emerge while taking oral contraception, but also while using progesterone products [8]. In practical terms, it is necessary to remember that prescribing any estrogen therapy (including COC) requires a close monitoring of the patient, since the same difficulties may occur as in pregnancy.
All currently available publications only review the casuistical correlation between OC and ICP. Retrospective analysis of a female patient population was presented by Williamson et al. [25], while no published randomized studies are available at present. In our paper we aim to elaborate on this area of expertise, drawing on our own experience with patients afflicted with ICP, who were repeatedly surveyed on their OC use.
MATERIALS AND METHODS
Population of women diagnosed with ICP underwent retrospective analysis in two time periods. The split of the population was limited by modern methods of intrauterine fetal status monitoring, which have been available in Trencin since 1992, and by introducing UDCA in the treatment of ICP in 1997.
Group 1 (no UDCA treatment applied) included patients who were initiated on hepatoprotective, dietary and regimen-specific measures, and administered antihistamines (between 1992 and 1996).
Group 2 included UDCA-treated patients (between 1997 and 2004). No hepatoprotective or antihistamine agents were administered. UDCA was administered orally at a dose of 500–750 mg per day, depending on the patient’s weight and her response to treatment. A dose higher than 750 mg per day was never administered in any patient.
The ICP diagnosis was made by a hepatologist. The presence of pruritus, supported by the elevated alanine aminotranspherase (ALT) or alkaline phosphatase (ALP) levels, was considered to be the evaluation criterion. It was not possible for us to investigate the serum bile acid levels during the observed time period. Only females initiated on active ICP treatment regimen and monitored for liver enzyme levels were included in the study. The patients with suspected ICP who delivered without a diagnostic assessment and initiation of therapy were not included in the research. Liver and gallbladder ultrasound exam was performed as well as a differential diagnosis of any other potential diseases with increased ALT. There was no evidence found of viral hepatitis in any of the women. All patients were consulted by a hepatologist and obstetrician about the nature and prognosis of their disease. The quality and scope of consultation would vary over time due to the methods of treatment accompanying the evolving disciplines of perinatology and hepatology.
In 2008, a questionnaire was sent to the women treated for ICP, which contained questions focused on their reproductive behavior, and progress of their and their children’s health condition (Appendix 1). A minimum interval of 3 years was selected to obtain the relevant data about the patients’ health condition and reproductive behavior. The use of OC and incidence of difficulties comparable to those with ICP was analyzed in the individual groups, as well as the presence of gallstones or cholecystectomy.
The file was subject to statistical analysis, which was coupled with the average, median, and standard deviation percentage analysis. The relationship of significance of the two categorical variables was expressed by the chi-square test of independence. The relationship between the numerical and categorical variable was analyzed by ANOVA.
RESULTS
Between 1992 and 2004, 79 women diagnosed with ICP gave birth. 36 pregnant women in Group 1 were analyzed between 1992 and 1996, and 43 pregnant women in Group 2 were analyzed between 1997 and 2004 (Table 1). The frequency of ICP occurrence within the two monitored time periods remained unchanged (0.41% and 0.42%).
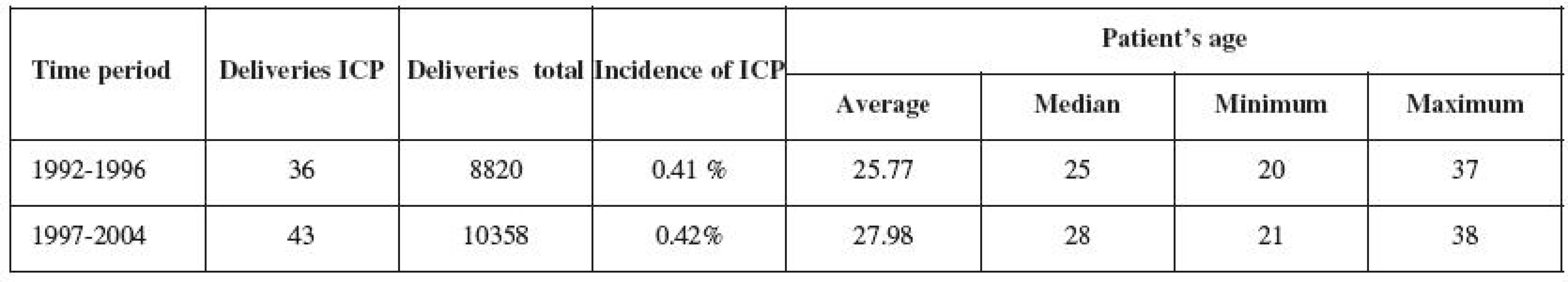
The questionnaire on reproductive behavior and development of health condition was sent to all women with ICP in 2008. The return rate was 71% and did not differ in either of the groups (p=0.81). The questionnaire analyzed 26 women with ICP (72.2%) in the first group and 30 women (69.8%) in the second group.
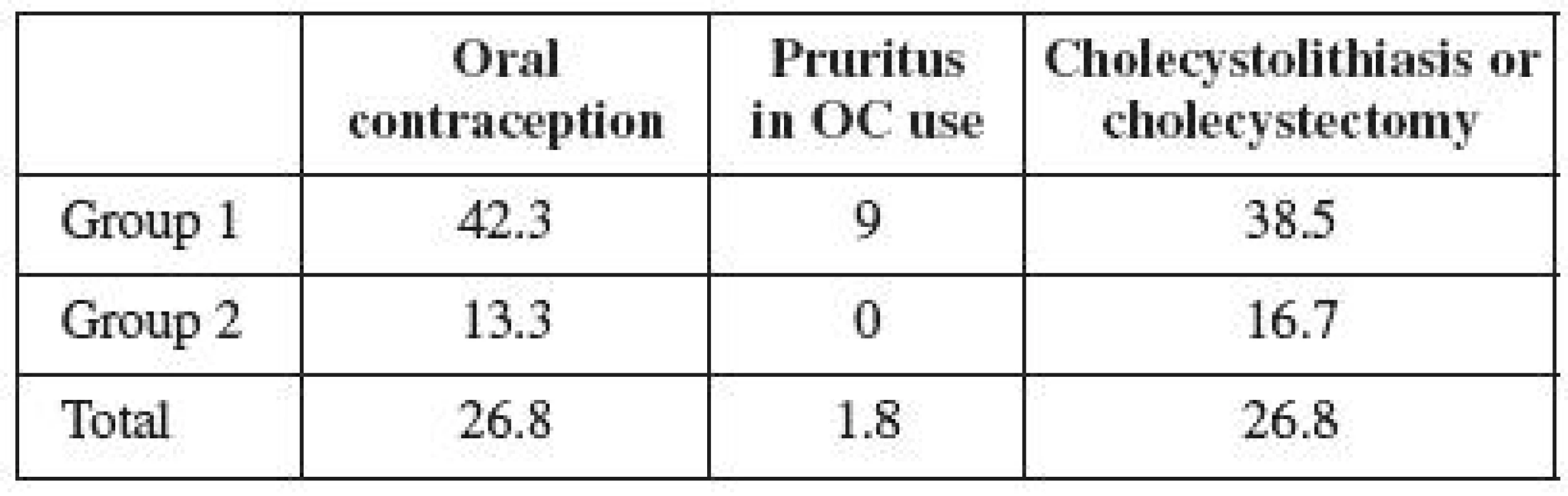
There were 15 users of hormonal contraception (26.8%) in the observed population of women with ICP (Table 2). However, 11 women (42.3%) used OCs in the group not treated with UDCA, and 1 woman (9%) developed pruritus while taking OCs. In the group treated with UDCA, only 4 women (13.3%) were taking OCs, and none of them developed pruritus while on the pills. The frequency of cholecystolithiasis or cholecystectomy occurrence was 26.8% in the entire observed population (Table 2). Its occurrence in the untreated group was observed in 10 women (38.5%), and in 5 women in the treated group (16.7%). The frequency of cholecystolithiasis occurrence in the 1992–1996 population of women was more than twice higher compared to the period of 1997–2004. The occurrence of cholecystolithiasis in both groups of OC users was observed in 4 out of 15 women (27%); its occurrence was observed in 11 (also 27%) out of 41 non-OC users. No correlation was therefore demonstrated between the use of contraceptives and the risk of cholecystolithiasis or cholecystectomy.
DISCUSSION
The frequency of ICP occurrence in Trencin region population is identical with the data published and valid for Central Europe. The gradual age increase in delivering women (Table 1) is not specific to the groups of ICP mothers, but is also noted in the general population. Similar demographic trends can be observed in other developed countries in Europe and North America [2, 12].
The questionnaire survey, length of time interval and analyzed data are unique within the world published papers [25]. Time lapsed from the analyzed pregnancies is 3 to 16 years in the specific cases included in our population. It is obvious that the time factor is an important aspect of the analysis and can, in some cases, significantly affect the results in individual groups.
Very few data in literature are available on the frequency of OC use in women with a history of ICP. Reproductive behavior of affected women may be influenced by potential concerns about hormonal therapy, as the supposition about relative contraindications of hormones taken by women with a history of ICP is still present [5, 6, 9, 15]. Only 7% of UK gynecologists recommend contraceptive preparations to their patients with a history of ICP [22].
The obtained results show that as many as one third of the women with a history of ICP were taking oral contraception. Much more frequent use of contraception is observed in the first group of women, who were not administered UDCA in pregnancy. The difference between the individual groups is statistically significant (p=0.015). Group 2 included the women treated in a modern way, who were more informed of their disease, too. This fact may presuppose their bigger concerns over the safety of hormone use; on the other hand, these women are younger and thus more inclined to OC use. Yet our assumptions were right about their fear of the hormone therapy use after overcoming liver disease. However, the size of the analyzed population, or possibly the way reproduction process continues in younger women may both be important. Epidemiological data presuppose that approximately one third of the Slovak female population uses hormonal contraception. Our Group 1 analysis reveals the use of contraception to be higher than the Slovak average. It is possible to conclude that a history of ICP has no influence on the decision of women in the first time period to use hormonal therapy. Our results show any potential concerns about the use of OC with a history of ICP to be exaggerated, of which both the patients and professional public should be informed.
ICP etiopathogenesis presupposes the occurrence of pruritus in women with a history of ICP who are using the pills [17, 23, 24]. Williamson et al. [25] has traced the incidence of pruritus during OC use in 14 % of women with a history of ICP. Our results reveal that only a small percentage of women experience pruritus during their use of contraceptives. The difference between both groups is not significant (p=0.533).
Family and genetic background of ICP and the discomfort of women affected during their use of hormonal contraception has been described in detail, using the example of a family where all five sisters suffered from symptoms of ICP during their pregnancy, or pruritus while using OCs [11]. Three of them underwent cholecystectomy because of cholecystolithiasis. Likewise, their mother had undergone cholecystectomy and had suffered the symptoms of ICP during all her pregnancies. A history of cholecystolithiasis was also found in the grandmother of the analyzed family. According to the published data, 10–20% of the adult population suffers from cholecystolithiasis in industrial countries [16]. The incidence of cholecystolithiasis in women aged 20 - 30 is estimated at 2–2,5%; however, it increases to 25–30% in women above 60 years [7]. The relative risk of cholecystolithiasis incidence is 3.7 in women with a history of ICP [21]. Cholecystolithiasis (or cholecystectomy) was found in 27% of our analyzed women. The difference between both groups was significant (p=0.043). Given that the incidence of cholecystolithiasis increases with age and reaches its top only after the age of 50, our data turn out to be consistent with the published information [10, 14]. So far, the younger women of Group 2 have suffered from cholecystolithiasis in 17% of cases. The incidence of disease in the first group has already exceeded the population rate. Its incidence in the entire population is consistent with the published data on ICP, and may be assumed to gradually increase over time.
CONCLUSION
The management of ICP patients has changed significantly since UDCA was introduced in the treatment as the first-line therapy. Data on oral contraception use in women with a history of ICP are remarkable. The use of oral contraception can be considered safe also based on our research results. Only a minimum of adverse effects have been observed at a relatively high percentage of women using OC therapy.
Peter Kaščák, MD, PhD.
Department of Obstetrics and Gynecology, General Hospital
Legionárska 28
911 71 Trenčín
e-mail: pkascak@gmail.com
Sources
1. Azzaroli, F., Turco, L., Lisotti, A., et al. The pharmacological management of intrahepatic cholestasis of pregnancy. Curr Clin Pharmacol, 2011, 6, 1, p. 12-17.
2. Benzies, KM. Advanced maternal age: Are decisions about the timing of child-bearing a failure to understand the risks? Can Med Assoc J, 2008, 178, p. 183-184.
3. Brites, D., Rodrigues, CM. Elevated levels of bile acids in colostrum of patients with cholestasis of pregnancy are decreased following ursodeoxycholic acid therapy. J Hepatol, 1998, 29, p. 743-751.
4. Brites, D., Rodrigues, CM., Oliveira, N., et al. Correction of maternal serum bile acid profile during ursodeoxycholic acid therapy in cholestasis of pregnancy. J Hepatol, 1998, 28, p. 91-98.
5. Connolly, TJ., Zuckerman, AL. Contraception in the patient with liver disease. Semin Perinatol 1998, 22, 2, p. 178-182.
6. Čepický, P., Cibula, D., Dvořák, K., et al. Doporučení k předpisu kombinované hormonální kontracepce (CC). Mod Gynek Porod, 2007, 16, Suppl. 1, s. 151-158.
7. Ehrmann, J. Játra a těhotenství. In: Ehrmann a kol. Ikterus – diferenciální diagnostika. Praha: Grada Publishing, 2003, s. 192‑196.
8. European Association for the Study of the Liver: EASL Clinical Practice Guidelines: Management of cholestatic liver diseases. J Hepatol, 2009, 51, 2, p. 237-267.
9. Faculty of Family Planning and Reproductive Health Care, Royal College of Obstetricians and Gynecologists. UK medical eligibility criteria for contraceptive use, 2005-2006. London: Faculty of Family Planning and Reproductive Health Care, 2006.
10. Geenes, V., Williamson, C. Intrahepatic cholestasis of pregnancy. World J Gastroenterol, 2009, 15, p. 2049-2066.
11. Hardikar, W., Kansal, S., Oude Elferink, RP., et al. Intrahepatic cholestasis of pregnancy: when should you look further? World J Gastroenterol, 2009, 15, p. 1126-1129.
12. Joseph, KS., Allen, A., Dodds, L., et al. The perinatal effects of delayed childbearing. Obstet Gynecol, 2005, 105, p. 1410-1418.
13. Joshi, D., James, A., Quaglia, A., et al. Liver disease in pregnancy. Lancet, 2010, 375, 9714, p. 594-605.
14. Kondrackiene, J., Kupcinskas, L. Intrahepatic cholestasis of pregnancy – current achievements and unsolved problems. World J Gastroenterol, 2008, 14, p. 5781-5788.
15. Lindberg, MC. Hepatobiliary complications of oral contraceptives. J Gen Intern Med, 1992, 7, 2, p. 199-209.
16. Mareček, Z. Cholelitiáza a ostatní nenádorové nemoci žlučového ústrojí. In: Mařatka, Z. Gastroenterologie. Praha: Univerzita Karlova, Nakladatelství Karolinum 1999, s. 345-362.
17. Meier, Y., Zodan, T., Lang, C., et al. Increased susceptibility for intrahepatic cholestasis of pregnancy and contraceptive-induced cholestasis in carriers of the 1331T>C polymorphism in the bile salt export pump. World J Gastroenetrol, 2008, 14, p. 38-45.
18. Milkiewicz, P., Elias, E., Williamson, C., Weaver, J. Obstetric cholestasis. Br Med J, 324, 2002, p. 123-124.
19. de Pagter, AG., van Berge Henegouwen, GP., ten Bokkel Huinink, JA., et al. Familial benign recurrent intrahepatic cholestasis. Interrelation with intrahepatic cholestasis of pregnancy and from oral contraceptives? Gastroenterology, 1976, 71, 2, p. 202-207.
20. Palmer, DG., Eads, J. Intrahepatic cholestasis of pregnancy: a critical review. J Perinat Neonatal Nurs, 14, 2000, p. 39-51.
21. Ropponen, A., Sund, R., Riikonen, S., et al. Intrahepatic cholestasis of pregnancy as an indicator of liver and biliary diseases: a population-based study. Hepatology, 2006, 43, p. 723-728.
22. Saleh, M., Abdo, K. Consensus on the management of obstetric cholestasis: National UK survey. Br J Obstet Gynaecol, 2007, 114, p. 99-103.
23. Stieger, B., Geier, A. Genetic variations of bile salt transporters as predisposing factors for drug-induced cholestasis, intrahepatic cholestasis of pregnancy and therapeutic response of viral hepatitis. Expert Opin Drug Metab Toxicol, 2011, 7, 4, p. 411‑425.
24. Valla, D., Benhamou, JP. Liver disease related to oral contraceptives. Dig Dis, 1988, 6, p. 76-86.
25. Williamson, C., Hems, LM., Goulis, DG., et al. Clinical outcome in a series of cases of obstetric cholestasis identified via a patient support group. Br J Obstet Gynaecol, 2004, 111, p. 676‑681.
Labels
Paediatric gynaecology Gynaecology and obstetrics Reproduction medicineArticle was published in
Czech Gynaecology

2011 Issue 5
-
All articles in this issue
- Follow-up after conservative surgery for cervical precancer lesions
- Basic anthropometric characteristics and their relationship with the metabolism of saccharides in women with PCOS
- Evaluation of initial experience with safety and short efficacy of mini-sling antiincontinence procedures MiniArc and AJUST system
- Axillary reverse mapping – chance to prevent lymphedema in breast cancer patients
- Current classification of malignant tumours in gynecological oncology – part II
- Thrombin generation test at physiological and risk gravidity
- Names and eponyms in Italy obstetrics and gynaecology
- Perineal audit: reasons for more than one thousand episiotomies
- Risk factors in the medical history of pregnant women undergoing congenital heart defect prenatal screening
- Fetal hypotrophy dopplerometry
- Single embryo transfer – possibilities and limits
- Hormonal contraception after intrahepatic cholestasis of pregnancy
- Czech Gynaecology
- Journal archive
- Current issue
- About the journal
Most read in this issue
- Fetal hypotrophy dopplerometry
- Current classification of malignant tumours in gynecological oncology – part II
- Single embryo transfer – possibilities and limits
- Perineal audit: reasons for more than one thousand episiotomies

