Occult invasive cervical cancer, FIGO stage III, with a negative Pap smear test – clinical presentation and brief review of the literature
Okultní invazivní karcinom děložního čípku, FIGO stadium III, s negativním Pap stěrem – klinický obraz a stručný přehled literatury
Okultní karcinom je vzácný a bývá diagnostikován náhodně po prosté hysterektomii. Při histologickém vyšetření je obyvkle zjištěno FIGO (International Federation of Gynecology and Obstetrics) stadium I, vzácně vyšší s negativními předoperačními diagnostickými testy, jako je např. Pap stěr. Popisovaný klinický případ je vzácným případem karcinomu děložního čípku diagnostikovaného v dobe hysterektomie s negativnim exo-endocervikálním Pap stěrem a diagnostickými testy jako je transvaginální ultrazvuk, magnetická rezonance břicha a výpočetní tomografie břicha při diagnóze degenerujícího myomu nebo suspektného karcinomu. Na operačním sále došlo ke změně radikality chirurgického zákroku a operace byla dokončena při odstranění obvyklých prvků a cervikálního prstence a provedení oboustranné pánevní lymfadenektomie. Finální histologické vyšetření indikovalo FIGO stadium III, pro které pacientka podstoupila radioterapii a chemotherapii.
Klíčová slova:
patologie – magnetická rezonance – výpočetní tomografie – okultní karcinom děložního čípku – klasifikace FIGO
Authors:
Paolo Meloni 1
; Terenzia Simari 2
; Roberta Lai 3
; I. Cassella 3
; Rodolfo Brizio 4
; Roberto Conturso 5
; D. Russo 6
; Edward Araujo Júnior 7
; Gabriele Tonni 8
Authors‘ workplace:
Department of Obstetrics and Gynecology, Imperia Hospital, ASL1 Imperiese, Italy
1; Gynecology Obstetrics Specialist Outpatient Clinic, ASL1 Imperiese, Italy
2; Radiology Service, Imperia Hospital, ASL1 Imperiese, Italy
3; Human Pathology Service, Imperia Hospital, ASL1 Imperiese, Italy
4; Obstetrics and Gynecology Service, Sant’Anna Clinic, Lugano, Switzerland
5; Department of Human Pathology, Cerbapath, Nice, France
6; Department of Obstetrics, Paulista School of Medicine – Federal University of São Paulo, São Paulo-SP, Brazil
7; Department of Obstetrics and Gynecology, and Researcher, Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS), ASL Reggio Emilia, Reggio, Emilia, Italy
8
Published in:
Ceska Gynekol 2024; 89(1): 34-39
Category:
Case Report
doi:
https://doi.org/10.48095/cccg202434
Overview
Occult cervical cancer is rare and is diagnosed incidentally after a simple hysterectomy. The staging upon histological examination is usually International Federation of Gynecology and Obstetrics (FIGO) stage I, rarely higher with negative preoperative diagnostic tests such as a Pap smear. The clinical case in question is a rare case of cervical carcinoma diagnosed at the time of hysterectomy with a negative exo-endocervical Pap smear, diagnostic tests including transvaginal ultrasound, abdominal magnetic resonance imaging and abdominal computed tomography with a diagnosis of degenerating myoma or suspected sarcoma. In the operating theatre, the surgical radicality was modified and the operation was completed with removal of the parameters, vaginal collar and bilateral pelvic lymphadenectomy. The final histological examination indicated FIGO stage III, for which the patient underwent radiotherapy and chemotherapy.
Keywords:
computed tomography – pathology – magnetic resonance imaging – occult carcinoma of the cervix – FIGO classification
Introduction
Occult invasive cervical cancer is diagnosed incidentally after a simple hysterectomy [1]. The diagnosis of cervical lesions is made after a cytological sample called a Pap smear. Patients with abnormal Pap smears undergo a second level of diagnosis, colposcopy, which involves taking a targeted biopsy for histological examination [2]. In some cases, there are discrepancies between the cytological and histological examination, hence a review of the slides is carried out.
Cytological lesions correlate with histological ones in the following percentages: low-grade lesions are often negative on further diagnostic investigation, for which atypical squamous cells (ASCUS) are negative in 80–90% of cases and low-grade squamous intra-epithelial lesions (L-SIL) in 60–70% of cases [3], with a very low risk of 0.2% of the presence of invasive neoplasia and a high risk of overtreatment [4,5]. Other high-grade cytological lesions, such as ASC-H-SIL, have an approximately 2% chance of being an invasive/microinvasive carcinoma [6]. If the lesion is adenocarcinoma of the glandular type, the histological correlation with squamous cell carcinoma is very high at around 54% [7].
Early-stage cervical cancer is usually diagnosed by colposcopy-directed biopsy and/or endocervical curettage, but some missed lesions need to be detected by conization because they are occult [8]. Treatment of invasive cervical cancer is influenced by the stage of the disease, which is based on clinical assessment. There is no standard treatment for stage Ib-IIa cervical cancer. Both radical surgery and radical radiotherapy have been shown to be equally effective, but differ in terms of associated morbidities and complications. There is much conflicting published work on the treatment of stage Ib-IIa large cervical cancer including: primary surgery followed by radio or postoperative radio/chemotherapy, and primary chemo-radiotherapy followed by radical surgery. Treatment decisions should be individualized and based on multiple factors, including disease stage, age, patient health status, tumor-related factors, and treatment preferences to achieve the best treatment with minimal complications [9]. Invasive cervical cancer discovered after simple hysterectomy remains a problem. Little is known about the best management of this group as there are no relevant outcome studies.
Postoperative radiotherapy is a good treatment for patients with cervical cancer who have undergone suboptimal simple hysterectomy. Appropriate selection criteria for further surgery need to be established.
In this article, we present a case of occult cervical cancer, FIGO stage III, with a negative Pap smear test, including both histopathological and imaging diagnostics.
Case Presentation
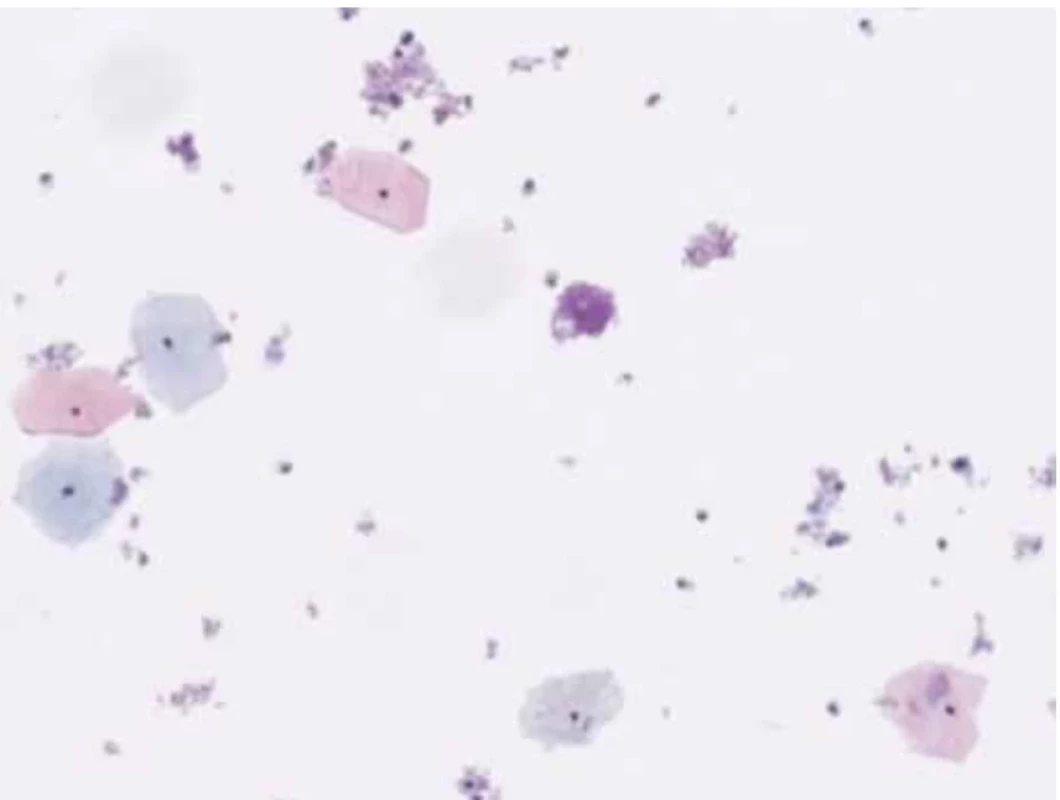
A 46-year-old female patient undergoes a check-up for pelvic pain. On examination, a voluminous formation was found in the posterior fornix of the vagina with involvement of the cervix. Upon examination with the speculum, the exocervix was observed without macroscopic lesions and the GSC was observed at the endocervical level. A thin-layer Pap smear was taken with the presence of exocervical and endocervical material. Microscopic analysis highlights the presence of numerous intermediate squamous cells and some superficial cells without atypia, hence the absence of squamous intraepithelial lesions or signs of malignancy (Fig. 1).
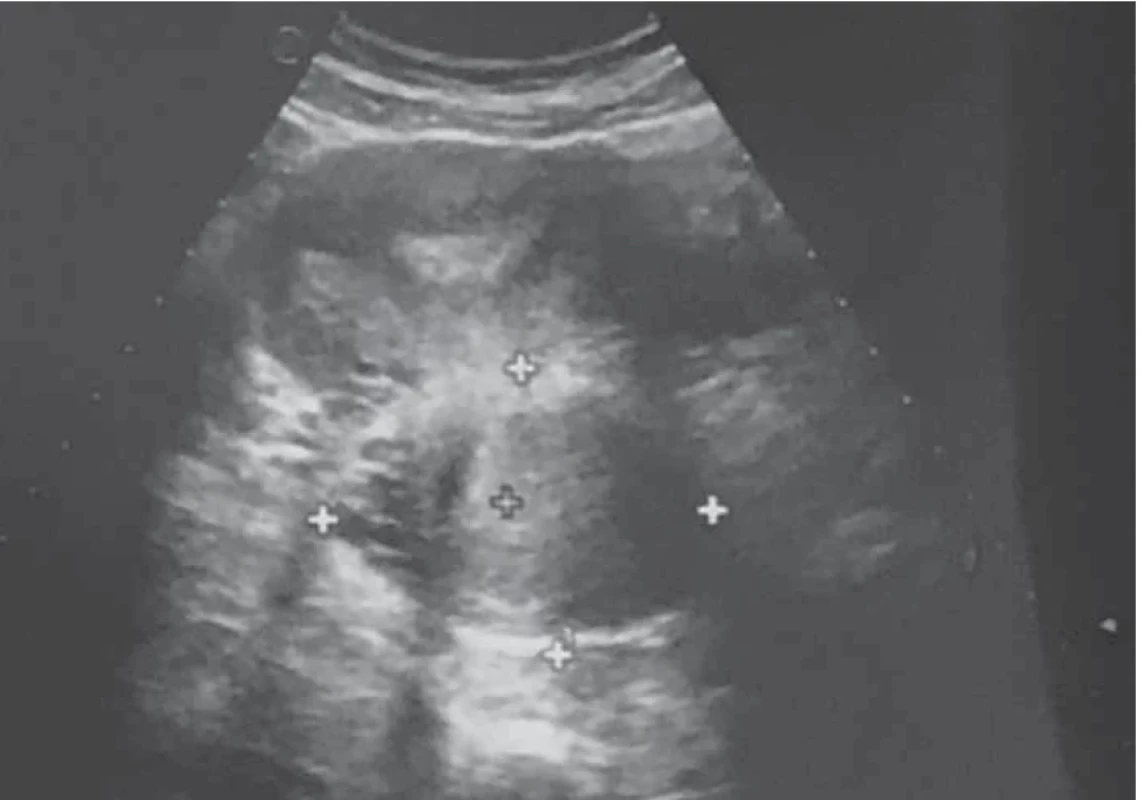
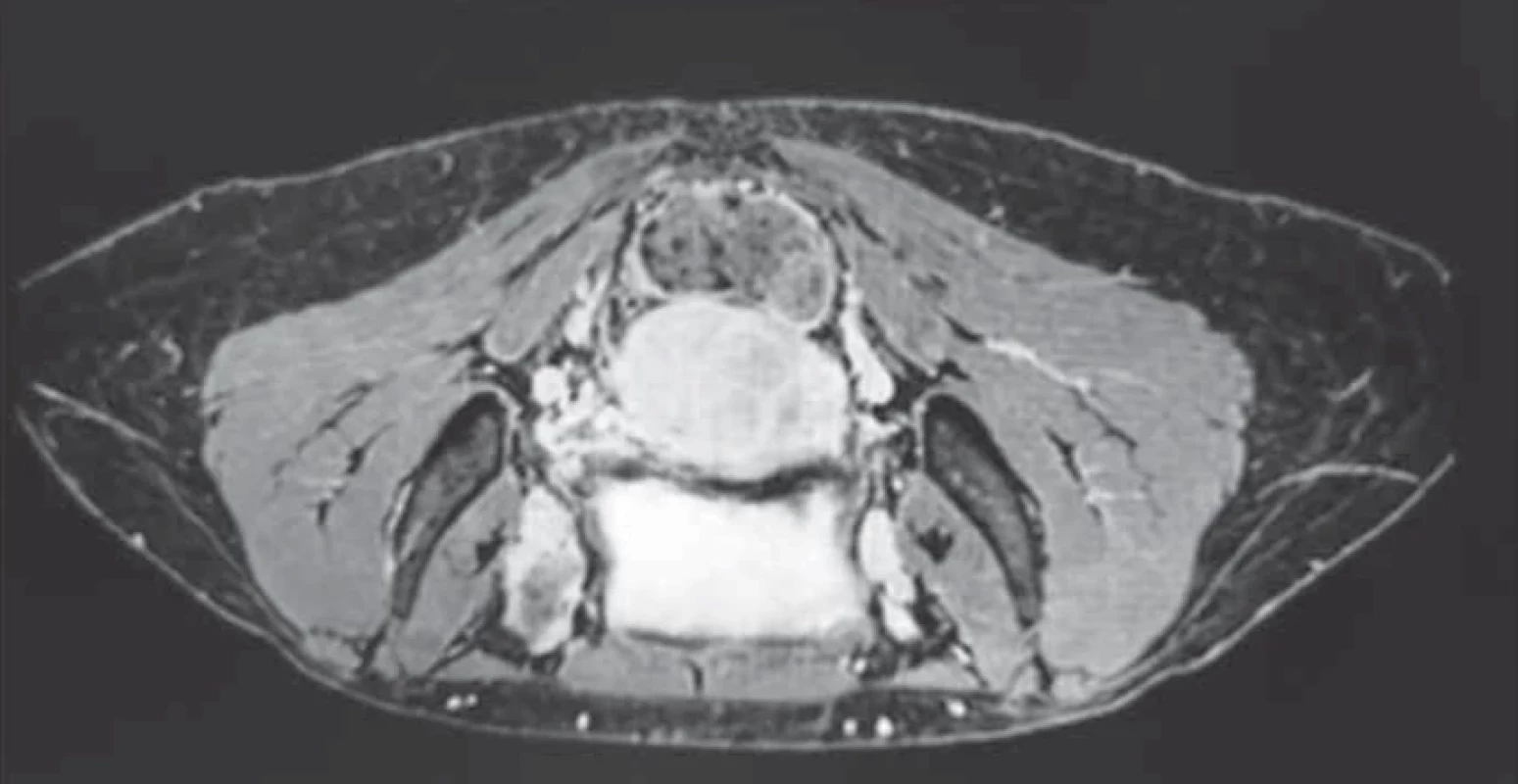
Upon evaluation with abdominal-pelvic ultrasound, a voluminous formation of approximately 4.5 cm was found in the intramural cervical subserosa with isthmic involvement. The described neoformation did not appear to involve the cervical canal on ultrasound (Fig. 2). The ultrasound appearance of the mass led to a presumptive diagnosis of sarcoma. Magnetic resonance imaging (MRI) of the pelvis, after administration of gadolinium-based minimum detectable concentration (MDC) demonstrated marked enhancement of the lesion, while the differential diagnosis between the degenerating myoma and uterine sarcoma remained challenging (Fig. 3).
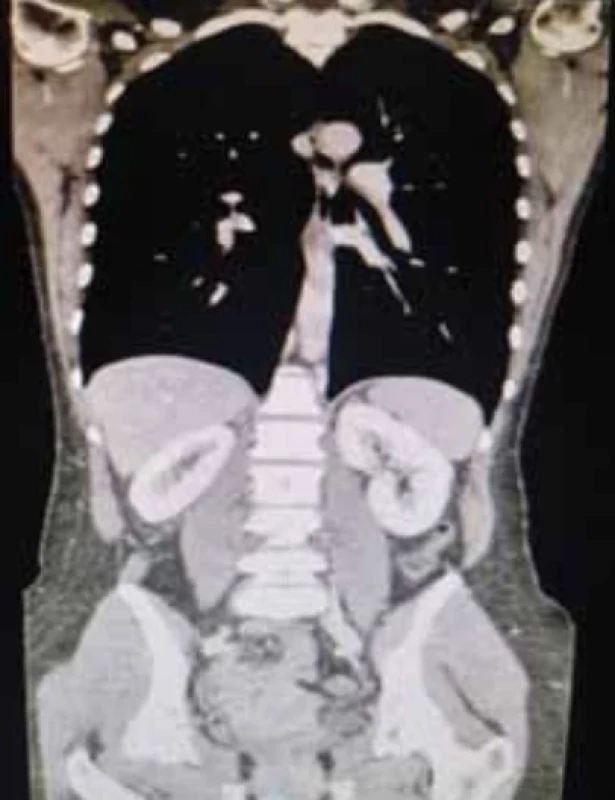
Computed tomography (CT) was performed with volumetric acquisition with direct scans and during rapid administration of intravenousiodinated MDC in the arterial, venous and late phases. There was non-homogeneous enlargement of the uterine portion of the cervix with discrete hypodense dilatation of the uterine lumen. There was a normal course of the ureters. No significant lumbo-aortic adenopathy was present along the course of the ileo-pelvic vessels. Ascites or peritoneal free-fluid was absent. CT confirmed the cervical neoformation, with the lymph node stations being negative (Fig. 4).
The patient underwent total hysterectomy with removal of the vaginal collar and simultaneous bilateral salpingectomy with preservation of the ovaries. During the operation, a lesion was observed in the left parametrium, as if due to infiltration, so it was decided to remove the parameters and the vaginal collar and send the material in for histological examination. The extemporaneous histological examination diag - nosed a squamous cell carcinoma measuring 4.5 cm. After a telephone consultation with members of the gynecological disease metabolic team, we proceed with bilateral pelvic lymphadenectomy as it is likely to be stage IA2, which requires radical abdominal hysterectomy and bilateral systematic pelvic lymphadenectomy (level of evidence B).
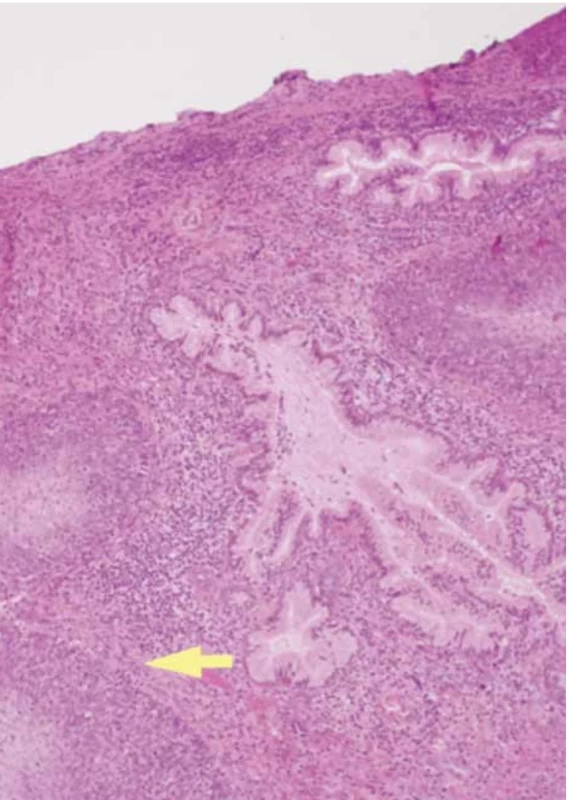
The final histological examination shows a macroscopic uterus with a 1.5 cm vaginal collar. The endometrium of the left cervical wall and cervical canal were deformed by a mural mass extending longitudinally for approximately 4.5 cm into the loose left paracervical tissue and vagina. The cervical canal had a regular surface. The cervix was prolapsed with edematous lips. Microscopic squamous cell carcinoma of the cervix (p40+ immunophenotype, GATA-3 negative) was p16 positive with comedonecrosis, moderately differentiated (G2), and keratinizing (Fig. 5). The neoplasm involved the cervix and entire cervical canal, with infiltration of the paracervical tissues and vagina.
Discussion
There is no consensus in the scientific literature on the management of occult carcinoma of the cervix. The reported studies refer to carcinomas diagnosed histologically after surgery and not during surgery. The case presented is an occult carcinoma of considerable size diagnosed intraoperatively. Some studies show that neoadjuvant chemotherapy does not improve the long-term overall survival of overweight patients with early cervical cancer compared with primary radical surgery [10]. A very important surgical procedure in the treatment of cervical cancer is parametrectomy. In the case of occult cervical cancer found after simple hysterectomy, radical parametrectomy with pelvic lymphadenectomy using minimally invasive surgery appears to be more effective than concurrent radiotherapy or chemoradiotherapy, with acceptable minimal morbidity observed [11]. In assessing the safety and efficacy of radical parametrectomy and radiotherapy or concurrent chemoradiotherapy for patients with occult invasive cervical cancer found after simple hysterectomy, although radical parametrectomy and radiotherapy/concurrent chemoradiotherapy have similar therapeutic efficacy, the lower rate of late complications observed with radical parametrectomy makes it preferable to radiotherapy/concurrent chemoradiotherapy [12].
The anatomical finding of suspected neoplastic infiltrations made it necessary to change the surgical radicality, from extrafascial hysterectomy to radical hysterectomy with removal of the parameters. The surgical procedure was favored by the preparation of the pararectal or posterior and paravesical or anterior oncological pits, which is always advisable to prepare, even if the operation is for a benign pathology. However, radical parametrectomy has risks and is not performed in early-stage cervical cancer because the incidence of parametrial involvement is very low. Based on these results, radical parametrectomy could be avoided in patients with very low risk parametrial involvement (tumor size ≤ 2 cm, no lymphovascular space invasion – LVSI, less than 50% stromal invasion, negative lymph nodes) [13].
Some studies have evaluated the morbidity and effectiveness of radical parametrectomy following extrafascial hysterectomy in patients with occult cervical cancer. Radical parametrectomy was found to be an acceptable option for patients diagnosed with occult cervical cancer at the time of extrafascial hysterectomy [14]. At the time of surgery, an extemporaneous histological examination was requested as there was a high level of suspicion that it was a carcinoma of the cervix rather than a sarcoma, and given the possibility that the patient would have to be re-operated, it was decided to look for the appropriate intervention immediately. The literature has reviewed patients with invasive cervical cancer found during a simple hysterectomy who underwent radical parametrectomy and upper vaginectomy with therapeutic lymphadenectomy; these studies show a greater overall survival in patients who underwent radical surgery [15].
The patient has undergone appropriate surgery with pelvic lymphadenectomy, as occult carcinomas must be treated in the same way as carcinomas diagnosed prior to hysterectomy [16]. The patient will undergo brachytherapy/radiotherapy and chemotherapy. In fact, patients with occult invasive cervical cancer discovered after a simple hysterectomy can be treated safely and effectively with salvage radiotherapy. In patients with no residual disease and a negative resection margin, intracavitary radiotherapy can be omitted [17]. Concomitant chemoradiotherapy is becoming a new standard in the treatment of advanced disease as it has been clearly shown to improve disease-free status, progression-free status and overall survival [8,18]. In addition, a meta-analysis evaluated the oncological outcomes of preoperative neoadjuvant chemotherapy followed by radical surgery versus radical surgery alone for the treatment of FIGO stage I–II cervical cancer. This meta-analysis showed that surgery after neoadjuvant chemotherapy for FIGO stage I–II cervical cancer and surgery alone had similar oncological outcomes [19]. Therefore, neoadjuvant chemotherapy did not improve long-term overall survival in obese patients with early cervical cancer compared with primary radical surgery [20].
The diagnosis of occult carcinoma of the cervix after hysterectomy is rare, especially in FIGO stage III. First-line treatment is platinum-based chemoradiotherapy and brachytherapy, but in this case, hysterectomy was performed. Radical surgery is an option especially in patients without negative risk factors: combination of tumor size, LVSI and/or depth of stromal invasion [16]. However, the quality of surgery, whether parametrectomy or lymphadenectomy, is critical in the management of large tumors. Therapeutic decisions, shared with the multidisciplinary gynecological oncology group, have been based on multiple factors, including stage of disease, age, medical conditions of the patient, factors related to the tumor and ongoing intervention, in order to achieve better treatment with minimal complications [9]. For these reasons, it is considered that an appropriate intervention has been carried out in relation to the clinical case, which must be followed by radiotherapy/chemotherapy treatment.
Submitted/Doručeno: 29. 3. 2023
Accepted/Přijato: 25. 9. 2023
Prof. Edward Araujo Júnior, PhD
Rua Belchior de Azevedo
156 apto. 111 Torre Vitoria
Vila Leopoldina
CEP 05089-030
São Paulo-SP, Brazil
araujojred@terra.com.br
Sources
2. Boselli F, De Martis S, Rivasi F et al. The Italian experience of a Pap test and speculoscopy based screening programme. J Med Screen 2000; 7 (3): 160–162. doi: 10.1136/jms.7.3.160.
3. Ho GY, Bierman R, Beardsley L et al. Natural history of cervicovaginal papillomavirus infection in young women. N Engl J Med 1998; 338 (7): 423–428. doi: 10.1056/NEJM199802123380703.
4. Arbyn M, Paraskevaidis E, Martin-Hirsch P et al. Clinical utility of HPV-DNA detection: triage of minor cervical lesions, follow-up of women treated for high-grade CIN: an update of pooled evidence. Gynecol Oncol 2005; 99 (3 Suppl 1): S7–S11. doi: 10.1016/j.ygyno.2005.07.033.
5. Arbyn M, Sasieni P, Meijer CJ et al. Chapter 9: Clinical applications of HPV testing: a summary of meta-analyses. Vaccine 2006; 24 (Suppl 3): S3/78–S3/89. doi: 10.1016/ j.vaccine.2006.05.117.
6. Katki HA, Schiffman M, Castle PE et al. Five--year risk of CIN 3+ to guide the management of women aged 21 to 24 years. J Low Genit Tract Dis 2013; 17 (5 Suppl 1): S64–S68. doi: 10.1097/LGT.0b013e3182854399.
7. Sharpless KE, Schnatz PF, Mandavilli S et al. Dysplasia associated with atypical glandular cells on cervical cytology. Obstet Gynecol 2005; 105 (3): 494–500. doi: 10.1097/01.AOG.0000152350.10875.02.
8. Massad LS, Perkins RB, Naresh A et al. Colposcopy standards: guidelines for endocervical curettage at colposcopy. J Low Genit Tract Dis 2023; 27 (1): 97–101. doi: 10.1097/LGT.000 0000000000710.
9. Kesic V. Management of cervical cancer. Eur J Surg Oncol 2006; 32 (8): 832–837. doi: 10.1016/j.ejso.2006.03.037.
10. Behtash N, Nazari Z, Ayatollahi H et al. Neoadjuvant chemotherapy and radical surgery compared to radical surgery alone in bulky stage IB-IIA cervical cancer. Eur J Surg Oncol 2006; 32 (10): 1226–1230. doi: 10.1016/j.ejso.2006.07.012.
11. Narducci F, Merlot B, Bresson L et al. Occult invasive cervical cancer found after inadvertent simple hysterectomy: is the ideal management: systematic parametrectomy with or without radiotherapy or radiotherapy only? Ann Surg Oncol 2015; 22 (4): 1349–1352. doi: 10.1245/s10434-014-4140-5.
12. Park JY, Kim DY, Kim JH et al. Management of occult invasive cervical cancer found after simple hysterectomy. Ann Oncol 2010; 21 (5): 994–1000. doi: 10.1093/annonc/mdp426.
13. Lu HW, Li J, Liu YY et al. Can radical parametrectomy be omitted in occult cervical cancer after extrafascial hysterectomy? Chin J Cancer 2015; 34 (9): 413–419. doi: 10.1186/ s40880-015-0041-7.
14. Leath CA 3rd, Straughn JM, Bhoola SM. The role of radical parametrectomy in the treatment of occult cervical carcinoma after extrafascial hysterectomy. Gynecol Oncol 2004; 92 (1): 215–219. doi: 10.1016/j.ygyno.2003.10.018.
15. Ayhan A, Otegen U, Guven S et al. Radical reoperation for invasive cervical cancer found in simple hysterectomy. J Surg Oncol 2006; 94 (1): 28–34. doi: 10.1002/jso.20550.
16. Cibula D, Pötter R, Planchamp F et al. The European Society of Gynaecological Oncology/European Society for Radiotherapy and Oncology/European Society of Pathology guidelines for the management of patients with cervical cancer. Radiother Oncol 2018; 127 (3): 404–416. doi: 10.1016/j.radonc.2018.03. 003.
17. Koh HK, Jeon W, Kim HJ et al. Outcome analysis of salvage radiotherapy for occult cervical cancer found after simple hysterectomy. Jpn J Clin Oncol 2013; 43 (12): 1226–1232. doi: 10.1093/jjco/hyt137.
18. Hosaka M, Watari H, Kato T et al. Clinical efficacy of paclitaxel/cisplatin as an adjuvant chemotherapy for patients with cervical cancer who underwent radical hysterectomy and systematic lymphadenectomy. J Surg Oncol 2012; 105 (6): 612–616. doi: 10.1002/jso.22136.
19. Yang SL, Chen L, He Y et al. Effect of neoadjuvant chemotherapy followed by surgery for FIGO stage I–II cervical cancer: a meta-analysis. J Int Med Res 2020; 48 (8): 300060520945507. doi: 10.1177/0300060520945507.
20. Li Y, Gong YX, Wang Q et al. Optimizing the detection of occult cervical cancer: a prospective multicentre study in China. Int J Womens Health 2021; 13 : 1005–1015. doi: 10.2147/IJWH.S329129.
Labels
Paediatric gynaecology Gynaecology and obstetrics Reproduction medicineArticle was published in
Czech Gynaecology

2024 Issue 1
-
All articles in this issue
- Preoperative and postoperative staging in endometrial cancer – a prospective study
- Importance of vaginal packing after laparoscopic sacrocolpopexy – retrospective study
- Discrepancies in staging and perioperative classification of pelvic endometriosis according to #Enzian 2021
- Pandemic fear and psychological resilience in pregnant women after the pandemic – a study in Turkey
- Occurrence of acute retrobulbar hemorrhage during birth
- Occult invasive cervical cancer, FIGO stage III, with a negative Pap smear test – clinical presentation and brief review of the literature
- Primary perineal endometriosis – a case report and literature review
- Ultrasound finding of endometrial polyp and factors increasing risk of malignancy
- Female orgasm, reproduction and couple relationships
- Uterovesical fistula and its treatment in Sub-Saharan Africa
- Birth plan – legal and medical aspects
- New recommendations for informing patients and gamete donors in assisted reproduction
- Diagnostika a léčba endometriózy
- Czech Gynaecology
- Journal archive
- Current issue
- About the journal
Most read in this issue
- Ultrasound finding of endometrial polyp and factors increasing risk of malignancy
- Diagnostika a léčba endometriózy
- Importance of vaginal packing after laparoscopic sacrocolpopexy – retrospective study
- Female orgasm, reproduction and couple relationships
